Radioactive decay rate depends on chemical environment
Radioactive dating is claimed to prove that the earth is billions of years old, but the methods are based on a number of unprovable assumptions. For example, it is assumed that radioactive decay rates have not changed in the past. Specifically, geochronologists assume that radioactive decay rates are unaffected by physical conditions like temperature and pressure. They also assume they are independent of the chemical environment.
The atomic nucleus is extremely tiny compared with the overall size of the atom—about 100,000 times smaller in diameter. Since the nucleus is located at the centre of the atom, it is well shielded by the surrounding electrons from external physical and chemical conditions. Radioactive decay, being a nuclear process, is thus considered to be independent of external conditions. The constancy of decay rate is a foundational assumption of the whole radioactive dating methodology. Faure states:
‘ … there is no reason to doubt that the decay constants of the naturally occurring long-lived radioactive isotopes used for dating are invariant and independent of the physical and chemical conditions to which they have been subjected …’1
One of the modes of radioactive decay, electron capture, occurs when a proton in the nucleus of an atom spontaneously captures an electron from one of the shells 2 and becomes a neutron.3 The mass of the atom remains the same but the atomic number decreases by one. Electron capture is the only radioactive decay mode that is recognised as possibly being affected by physical conditions such as pressure, but the effect is considered insignificant and is ignored.1
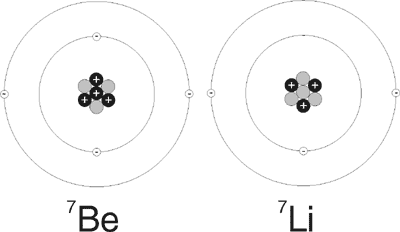
However, a recent paper about the decay of beryllium-7 (7Be) has found that, contrary to previous thinking, the chemical environment noticeably affects the half-life of radioactive decay by electron capture.4 Beryllium is a rare, hard, light metallic element in the second column of the periodic table—an alkaline earth element. Its nucleus contains four protons, and the usual stable form also contains five neutrons, and thus has a mass number of nine. There is a lighter isotope of beryllium with a mass number of seven, with only three neutrons in its nucleus. The lighter isotope is unstable and decays to Lithium-7 (7Li) by electron capture (Figure 1). The energy released in this process is mostly emitted as a gamma ray. The half life of 7Be is about 53 days.
In the recent paper, geochemist Chih-An Huh reported that the decay rate of 7Be depends on its chemical form.4 The measurements were done at the unprecedented high precision of ±0.01%, some ten times better than any reported previously. An extremely sensitive and stable spectrometer was used to monitor gamma rays from the decay of 7Be. Three different chemical forms of 7Be were measured, the hydrated Be2+ ion in solution surrounded by four water molecules ([Be(H2O)4]2+), the hydroxide (Be(OH)2), and the oxide (BeO). The measured half lives were 53.69 days, 53.42 days and 54.23 days respectively—a 1.5% variation from the shortest to the longest. The variation is much greater than previously considered.
Creationists, for many years, have disputed the billions of years from radioactive dating calculations because they conflict with the 6000-year Bible time-scale. One assumption they have challenged is the constancy of decay rates. Curiously, Richard Kerr has picked up this scepticism in his report of Huh’s findings, and makes a particular point of addressing creationists:
‘Creationists hoping to trim geologic history to biblical proportions will be disappointed—the variations seen so far are much too small, just a percent or so, to affect the Earth’s overall time scale.’5
Despite these comments, the 1.5% variation in the half-life of 7Be due to chemical environment was a surprise, and shows that the previous assumption that rates are constant is not correct. One of the most widely used geological dating methods, the radioactive decay of 40K to 40Ar, nearly always occurs by electron capture.6 The effect of chemical environment on the decay rate for 40K should be less than for 7Be because potassium has extra electrons in outer shells. These electrons would shield those inner electrons that are more vulnerable to electron capture from the external chemical environment. The important question, though, is what factors may have controlled the distribution of radioactive isotopes within the rocks of the earth.
Creationists have good reason to believe there is something wrong with the explanation that isotopes are due to billions of years of radioactive decay.7 This is not a blind faith—there are scores of geological evidences indicating that the earth is young.8 Changes in decay rates are only one possible explanation and will probably not be the complete answer. Many other factors need to be investigated. For example, we need to explore how isotopes behave deep within the earth during partial melting, and also in magma-rock systems during crystallisation. Creationists are actively investigating these and other pertinent areas as time and funds allow.9
References
- Faure, G., Principles of Isotope Geology, 2nd ed., John Wiley & Sons, New York, p. 41, 1986. Return to text.
- Only from the s orbitals, because all others have nodes at the nucleus, i.e. regions of zero probability of finding an electron. Return to text.
- An electron-neutrino is also released. Return to text.
- Huh, C.-A., Dependence of the decay rate of 7Be on chemical forms, Earth and Planetary Science Letters 171:325–328, 1999. Return to text.
- Kerr, R.A., Tweaking the clock of radioactive decay, Science 286(5441):882–883. Return to text.
- Faure, Ref. 1, p. 30. Return to text.
- John Woodmorappe, The Mythology of Modern Dating Methods, Institute for Creation Research, El Cajon, 1999. Return to text.
- See for example, Morris, J., The Young Earth, Creation-Life Publishers, Colorado Springs, 1994; Snelling, A., Radioactive dating failure: recent New Zealand lava flows yield ‘ages’ of millions of years, Creation 22(1):18–21, 1999; see also his technical paper, The Cause Of Anomalous Potassium-Argon ‘Ages’ for recent andesite flows at Mt Ngauruhoe, New Zealand, and the Implications for Potassium-Argon ‘Dating’. Return to text.
- Vardiman, L., RATE group prepares status report; ICR Impact 314, Institute for Creation Research, El Cajon, California, 1999. RATE is derived from Radioisotopes and the Age of the Earth, and is an inter-disciplinary group of six creationist scientists formed to investigate the radioisotope data from a young-earth perspective (see recommend products). Recently the group announced a five-year research programme estimated to cost some US$500,000. Return to text.



Readers’ comments
Comments are automatically closed 14 days after publication.