Journal of Creation 16(1):51–53, April 2002
Browse our latest digital issue Subscribe
Problem of short-lived radionuclides: design perspective
What’s the problem?
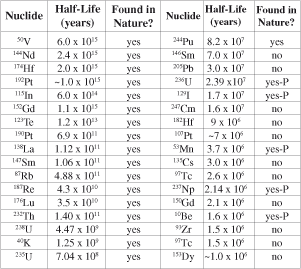
One of strongest alleged ‘proofs’ of a billions-of-years-old Earth is the absence in nature of radionuclides with half-lives much shorter than this—short-lived radionuclides (SLRNs). The argument is clearly described in the following statement from an atheistic anti-creationist journal:
‘Only 7 [SLRNs] are actually found. If the earth were only 10,000 years old, there should be detectable amounts of all 47 in nature because 10,000 years is not enough time for them to decay totally … [yet] all 17 nuclides with half-lives longer than 50 million years are found in nature.’1
The details are given in Table 1.
Assumptions
However, like all arguments about age, this is based on certain assumptions about the past. This assumes that the elements existed in the first place, but is there any reason to believe this? The Biblical Creation model does not preclude God from having created all elements in different quantities. It also assumes that the rate of nuclear decay has always been constant. So, we will address each assumption in turn.
Design perspective
When creating radioactive nuclides, God could be guided by the fact that SLRNs are highly radioactive, and would be dangerous to people and animals present on a young Earth. Therefore, it would be plausible to assume that He either created such nuclides in very small quantities or that He did not create them at all. There are several reasons for this
- SLRNs have high special activity (activity of 1 gram of nuclide), which grows with decrease of half-life:
- A lot of these radionuclides emit γ-quantums with high and hazardous energy.
- There is a strong correlation of short half-life with energy of emission, described by the standard Gamow theory of alpha decay involving quantum mechanical tunneling. SLRNs, therefore, would have emitted very dangerous radiation had they been created near people.3
- The compounds formed from these nuclides are often very soluble,4 so they would be leached easily from parent rocks and geochemically concentrated into biologically hazardous ‘hot spots’. Such agglomerations could occur readily during the Flood.

Decay rate
Click here for larger view
Recent research shows that decay rates were probably greater at some time (or times) in the past. Gentry shows that a possible explanation for 218Po radiohalos having no evidence of their mother elements, is a greater decay rate in the past.5,6 Also, the RATE group of creationist physicists and geologists has cited evidence for accelerated decay rates at certain times in the past, e.g.7–9
- The presence of daughter isotopes along the entire decay chain in proximity to parent isotopes.
- Visible scars (radiohalos) from alpha decay, in particular in halos with multiple rings that require much decay of 238U and its daughter elements, but the absence of mature halos in Phanerozoic rocks.
- The presence of the alpha particles themselves (helium nuclei) still within the rock where they were apparently formed by nuclear decay. The diffusion rate of helium through minerals would suggest that it would have escaped if the rocks were really billions of years old.
- Visible tracks from decay by fission.
- Residual heat produced by nuclear decay near high uranium concentrations is consistent with a pulse of accelerated nuclear decay.
There are theoretical means of producing accelerated decay, e.g. a small change in the fundamental constants or the shape of the nuclear potential well can have a large effect on the decay rate (but little effect on radiohalo diameter). Also, stripping atoms of electrons to leave a bare nucleus has been demonstrated to accelerate beta decay by a factor of a billion.10–12
The RATE researchers favour a pulse of accelerated decay rate during Creation Week, and possibly a smaller pulse during the Flood year. In any case, this points to higher radionuclide activity in the past which would be even more hazardous.
All these reasons are complementary. For example, a higher decay rate in the past would also mean that smaller quantities were initially created and also that SLRNs disappeared quicker out of the Earth’s surface (Figure 1).
Applying these principles to observations

a. creation in large quantity, modern decay rate;
b. creation in small quantity, modern decay rate;
c. creation in large quantity, greater decay rate in past;
d. creation in small quantity, greater decay rate in past.
Now we can consider what we observe in nature. It is well known, that there are four radioactive decay families: 232Th to 208Pb, 237 Np to 209Bi, 238U to 206Pb and 235 U to 207Pb. Among them, nuclides of the 235U to 207 Pb chain are found in small quantities (only 0.715% of naturally occurring uranium is 235U) and 237Np to 209Bi is absent. Long agers explain that over 4.5 Ga, 237Np (T½ = 2.1 Ma) and its daughters are completely decayed. To explain extant ratios of 235U, they assume that it originally comprised 23.6% of naturally occurring U.
Now let’s look at how this picture may be explained from a design perspective. We have at least five reasons for the 237Np chain being created in very small quantities or not at all. 235U (T½ = 700 Ma) has a lower specific activity than 237Np, and that is why 235U could have been created in small quantities.
Also, there are weighty reasons for how all created radionuclides existed in the beginning in equilibrium. Their initial quantities could have been such that their future decay rate were compensated by accumulation, and the following ratio would act:
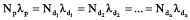
or
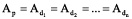
where: Np and Nd are the atom quantities of ‘parent’ and ‘daughter’ radionuclides; Ap and Ad are their activities; lp and ld are their decay constants.
This would bring constancy to the total activity on all the Earth’s surface, i. e:
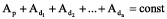
For instance, let’s consider the 235U decay chain. If it was originally created without its daughters, then its initial activity would increase twice in 6 days (Figure 2), because of the accumulation of the short-lived 231Th daughter (T½ = 25 hours). It could be quite dangerous if 231Th escaped into the biosphere and accumulated near certain areas of uranium with a high fraction of 235U. But for 238U, this decay would happen only in 300 days.
The absence of transuranium nuclides in the Earth’s crust can be explained in the same way. However, some of them have been found. It was Seaborg13 who first managed to scavenge 239Pu (T½ = 24 thousands years) out of pitchblende. Only 1 part per 1014 parts were found in the concentrate. He explains that this radionuclide could have been generated from 238U by bombardment of neutrons as follows:
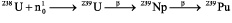
Possible sources of these neutrons include cosmic rays and spontaneous fission.14
This phenomenon is appropriate for our model, because small quantities of 239Pu acting as parent for 235U via alpha decay can maintain the constant activity of the entire decay chain. These principles are also applicable for other natural transuranic elements, such as 237Np.

SLRNs that are not members of these four radioactive families could be created in larger quantities, depending on their half-lives, because they do not have such long decay chains. Also, their activity could be maintained by different sources such as the case with the famous 14C (T½ = 5,700 years) produced by cosmic bombardment of 14N. Also, the lesser known 129I (T½ = 17 Ma) is assumed to be produced by fission and is estimated to be over 300 Ma old:
‘In the case of the Anadarko basin, the host formations are all Paleozoic, thus the age of 129I contained in the organic matter, which lived, died and accumulated in Paleozoic, is at least 300 Ma. This means that cosmogenic (surface) 129I component decayed to insignificant levels long ago … The most likely source for the 129I measured in these brines is fissiogenic … the most likely source for I is the Upper Devonian–Lower Mississippian Woodford Shale.’15
Probably, the explanation for 129I can be both its recent creation in small quantities and secondary sources. It’s important to note from this that long-agers would rather propose an unobserved source for an SLRN than concede that the rock is much younger than claimed.16 But if long agers can use the absence of something (i.e. an argument from silence) as proof of their view, how much more can creationists use the presence of something as disproof. This is especially so with detectable 14C activity in samples claimed to be millions of years old.17–20
Conclusion
On the basis of the above, a creationist model of SLRNs can comprise:
- Creation of radionuclide decay families in an equilibrium state.
- Initial absence or creation in small, safe quantities of radionuclides with half–lives less than 50 Ma.
- Creation of additional sources for generation of the total activity of the radionuclides on the Earth’s surface to be kept constant.
It should be noted that this model can work only in pre-Flood geology, which completely differs from post-Flood geology.
This model, of a recent creation of radionuclides in equilibrium, partly explains today’s observed U/Pb, Ru/Sr and other ratios used as ‘dating’ methods.
References
- Freske, S., Evidence for supporting a great age for the Universe, Creation/Evolution 1(2):34–39, 1980. Return to text.
- Dalrymple, G.B., The Age of the Earth, Stanford University Press, Stanford, p. 377, 1991. Return to text.
- Sarfati, J., Personal communication, 16 March 2000. Return to text.
- Woodmorappe, J., The Mythology of Modern Dating Methods, Institute for Creation Research, El Cajon, 1999. Return to text.
- Gentry, R.V., Radiohalos in a radiochronological and cosmological perspective, Science 184:62–66, 1974. Return to text.
- Gentry, R.V., Creation’s Tiny Mystery, 3rd Ed., Earth Science Associates, Knoxville, TN, 1992. Return to text.
- Vardiman, L., RATE group prepares status report, Impact 314:1, 1999. Return to text.
- Vardiman et al., Radioisotopes and the Age of the Earth, Institute for Creation Research, El Cajon, and Creation Research Society, St Joseph, 2000. Return to text.
- Oard, M., Rating radiodating [review of Ref. 8], JOC 15(2):31–32, 2001. Return to text.
- Takahashi et al., Bound-state beta decay of highly ionized atoms, Physical Review C36(4):1522–1527, 1987. Return to text.
- Jung et al., First observation of bound-state b–decay, Physical Review Letters 69(15):2164–2167, 1992. Return to text.
- Woodmorappe, J., Billion-fold acceleration of radioactivity demonstrated in laboratory, JOC 15(2):4–6, 2001. Return to text.
- Seaborg, G., The Transuranium Elements, McGraw-Hill Book Co., NY, 1949. Return to text.
- Hyde, E., The Nuclear Properties of the Heavy Elements, Prentice-Hall, Inc., Englewood Cliffs, 1969. Return to text.
- Moran, J., Origin of iodine in the Anadarko Basin, Oklahoma: an 129I study, American Association of Petroleum Geologists Bulletin 80(5):685–694, 1996. Return to text.
- Woodmorappe, Ref. 4, p. 26. Return to text.
- Snelling, A.A., Conflicting ages of Tertiary basalt and contained fossilised wood, Crinum, Central Queensland, Australia, JOC. 14(2):99–122, 2000; Radioactive Dating in Conflict, Creation 20(1):24–27, 1998 (popular version). Return to text.
- Snelling, A.A., Dating Dilemma: Fossil wood in ancient sandstone, Creation 21(3):39–41, 1999. Return to text.
- Snelling, A.A., Geological conflict: Young radiocarbon date for ancient fossil wood challenges fossil dating, Creation 22(2):44–47, 2000. Return to text.
- Giem, P., Carbon-14 content of fossil carbon, Origins, 51:6–30, 2001. Return to text.


Readers’ comments
Comments are automatically closed 14 days after publication.