Journal of Creation 33(2):84–92, August 2019
Browse our latest digital issue Subscribe
Destructive parasites: expressions of God’s creation?
Accounting for the presence of blood-sucking insects that transmit serious diseases is a challenging task. Propositions for the emergence of the malaria organism and filarial worms are suggested in this paper. It is argued that blood-sucking insects originally fed on nectar, honeydew, and perhaps other insects. Changes in gene expression conceivably led to utilization of pain-feeling animals as a ready source of nourishment for egg maturation, a function provided originally by mainly plant sources. Gene expression alterations, changes in insect vectoring of microbes, and their relationship with their hosts occurred after the Fall. It appears that as a result of such changes some benign or beneficial relationships were transformed into ones that caused harm. A study of one filarial worm indicates that it conceivably developed from nematode symbionts of the flies that transmit it. The origin of the malaria organism may represent a modification of some of the blood-associated organisms of frogs enabling an extension of host range. It is conceivable that these organisms operated at creation in a balanced and beneficial manner. Parasitism arose through gene loss, the expression of frontloaded genes now facilitating pathological life cycles, or other scenarios. Further progress can be made in constructing more definitive answers when greater knowledge accumulates.

When some look at the natural world and focus on the blighting diseases seen in plants and animals and the blinding of helpless children by destructive worms (filarial) they scorn at the notion of God. Notice the following from noted evolutionist Sir David Attenborough by way of illustration.
“When Creationists talk about God creating every individual species as a separate act, they always instance hummingbirds, or orchids, sunflowers and beautiful things. But I tend to think instead of a parasitic worm that is boring through the eye of a boy sitting on the bank of a river in West Africa, [a worm] that’s going to make him blind. And [I ask them], ‘Are you telling me that the God you believe in, who you also say is an all- merciful God, who cares for each one of us individually, are you saying that God created this worm that can live in no other way than in an innocent child’s eyeball? Because that doesn’t seem to me to coincide with a God who’s full of mercy.’”1
Transformations from a cooperative enterprise
Believers in the biblical account in general understand that God created the basic types of plants, animals, and lower forms of life. Undoubtedly, pre-Flood variants developed. At the time of the Flood, the basic or representative animal types were preserved rather than all species on account of the carrying capacity limitations of the rescue structure (here it must be acknowledged that we have no specific information on the preservation of insects). The enormous variation now seen among insects means that considerable changes have occurred in subsequent years as a consequence of adaptation and other phenomena.
With this pattern in mind, some suggestions will be given on the emergence of disease-carrying insects and two destructive parasites attacking humans. As readers will appreciate, there are considerable gaps in knowledge, but it is perhaps useful to attempt to construct a scenario that reasonably explains the world about us. I do not presume to provide all the answers.
Ecology of blood-sucking insects
A range of blood-sucking insects is involved in disease transmission. These include mosquitoes, flies, fleas, bugs, vampire moths, ticks, and mites. Two groups of blood-feeding insects, black flies (Simuliidae) and mosquitoes, will be considered. Black flies are transmitters of the river blindness nematode (Onchocerca volvulus). Mosquitoes are famous for their ability to carry the malaria parasite (Plasmodium spp.), a highly destructive disease. It is estimated that almost half of the world’s population is at risk of the disease, which can cause debilitation and death.2
A number of issues face blood-feeding insects, which will be addressed in brief.
Host finding
The ability of biting insects to find their host depends on multiple factors. Fluctuating levels of carbon dioxide are important clues to the presence of a human host for a mosquito such as Aedes aegypti. The insect flies along the fluctuating concentration plume and on approaching the host is assisted by visual cues and skin odourants. The landing is facilitated by the change in heat and humidity near the target. However, the context in which the stimuli occur will determine their significance.3 Feeding of Anopheles stephensi on moth larvae seems to be facilitated by an ability to detect a carbon dioxide gradient and other cues.4
Black flies also require a carbon dioxide cue to optimize their chances of contact with a host. Visual cues are of marginal value.5 It is considered that the highly specific black flies probably also detect subtle odours associated with the host.6
Feeding activities
Mosquitoes
The life cycle of mosquitoes is relatively simple with the adults laying eggs on water or damp surfaces prone to flooding, where they hatch to give larvae (wrigglers). The larvae feed on microorganisms and organic matter. Moulting occurs several times to give pupae from which the adult finally emerges at the surface of the water. The adults and larvae represent the feeding stages.7
Adults possess a flexible proboscis that is suited to penetrating soft surfaces, such as of fruit and skin. The basic food of both adult male and female mosquitoes is nectar and honeydew. Other sources are damaged fruit and leaves, tree sap, sugar cane trash, and regurgitated liquid from ants. Mosquitoes in the genera Toxorhynchites, Topomyia, and Malaya possess mouthparts that cannot pierce vertebrate skin and do not take a blood meal.8 Mosquitoes may feed on caterpillars, cicadas, and small dipterans and develop viable eggs. Even though mosquitoes (Aedes aegypti, Culex tarsalis, and Anopheles stephensi) may feed on insect larvae and produce viable eggs, such a meal is inferior to blood.4,9 The feeding behaviour on caterpillars and insects (figure 1) may help to explain their transition to blood-feeding activities on mammals, if it is assumed that they were not already facultative blood-feeders before the Fall. Insect haemolymph-feeding may have provided the opportunity for mosquitoes to come into contact with a suite of microbes that were able to exert a transformative effect on them.
The possession of a proboscis does not mean the mosquito population uses it to draw blood. Not all female mosquitoes require a blood meal to mature eggs for the first time. In these mosquitoes subsequent batches require a protein-rich meal.10
Black and deer flies
Black flies and deer flies are carriers of a number of diseases, but the one primarily focused on here is river blindness. African eye worm and its insect carrier, deer fly, will be mentioned as information allows.
Black fly adults lay eggs in aquatic habitats. These hatch giving rise to larvae that adhere to surfaces and consume a variety of small food resources (bacteria, algae, small aquatic animals, organic material) present in the moving streams. A non-feeding pupal stage follows and adults emerge within days of eggs being deposited. Adults emerging are winged with most adult females requiring a blood meal before eggs can mature.11 Deer fly larvae develop in high water content areas and feed on organic matter in the soil.12
Females of some deer and black fly species can mature a batch of eggs before a blood meal is consumed (autogenous species). Selected deer fly species can satisfy their blood meal requirements from reptiles and birds.13 Information on deer flies is rather limited meaning that hereafter our focus will be on black flies and river blindness rather than on African eye worm. Adult black and deer flies consume nectar and honeydew and the latter rotting fruit.14
Digestion
Blood-feeding brings with it a number of issues. First, the blood must be digested. Examination of the proteins present in saliva of blood-sucking insects indicated that the majority do not have a recognizable function. For components that do have a known function, the major targets are to inactivate elements in the blood-clotting cascade, inhibit platelet activation, and scavenge substances capable of causing pain, itching, and oedema. These features are shown also following an analysis of the salivary gland contents of male mosquitoes (do not feed on blood) found in those genera feeding on blood. They lack many compounds found in females and possess smaller amounts of components that function to dampen coagulation, platelet activity, and inflammation.15 Each group of insects possesses its own unique group of protein molecules that allow them to function. However, in all cases, common host defences are targeted.16 The most significant enzyme components identified in a number of blood-feeding insects are serine proteases and their inhibitors, hyaluronidases, and apyrase.17
Ovarian development
Mosquitoes
Steroid hormones (ecdysteroids) are intimately connected to initiation of yolk formation (vitellogenesis) and egg maturation.18 When mosquitoes are fed ecdysteroids, then egg development involving protein synthesis is stimulated in the absence of a blood meal. Similar compounds may be available in plants on which mosquitoes feed.19 Again, this gives some credibility to the suggestion that plant or insect feeding was the original food source for mosquitoes.
Black flies
Again, steroid hormones (particularly 20-hydroxyecdysone) are important to the maturation of eggs. In black flies, levels rise as eggs develop and also rise following blood-feeding, as they are synthesized from dietary cholesterol.20 Plants have the ability to synthesize 20-hydroxyecdysone (at least 78 families). The steroid is structurally identical to that found in insects and has no known function in plants except possibly as a deterrent to insects.21 Sterols found in plants and fungi can be used by insects to synthesize ecdysteroids.22 Overall, this allows the suggestion to be forwarded that originally black flies may not have needed to consume a blood meal to mature eggs, but rather existed on insect pupae or even on plants containing an adequate supply of ecdysteroids and protein.
Relationship of disease-causing organisms with their vectors
The relationship of disease agents and their vectors is complex and usually involves a number of stages.
Mosquitoes
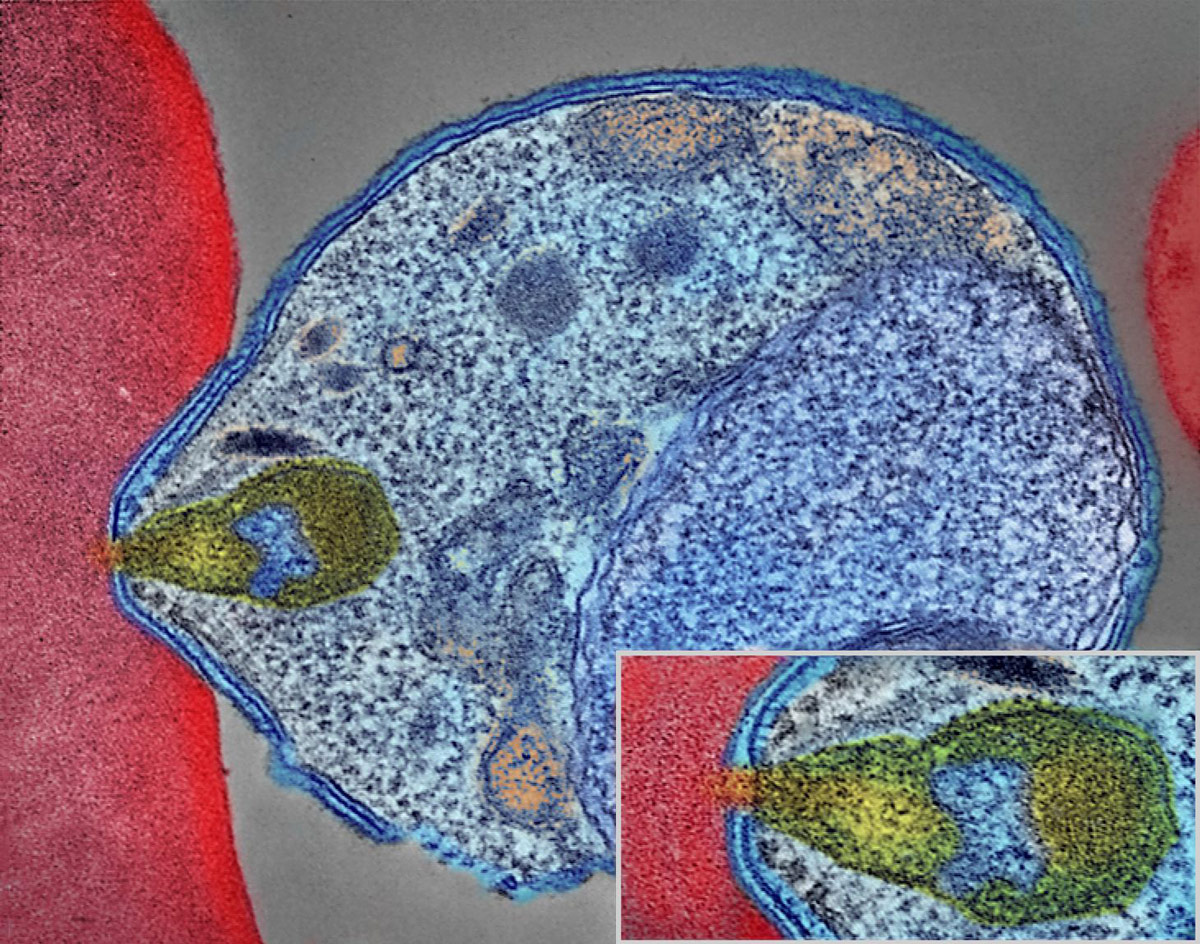
The malaria parasite is introduced into the bloodstream through saliva of the mosquito containing cells (sporozoite stage) of the parasite. These cells enter the liver and asexually multiply. When the merozoites are released they attach to red blood cells and penetrate (figure 2). In the blood cells growth and multiplication occurs that eventually results in their lysis. The released parasite can enter other red cells to continue the cycle. The lysis of red cells not only releases parasites, but toxins and debris that cause fever development. Occasionally, a different sequence of events happens in the red blood cells where two types of parasite cells are formed that do not rupture the red cells. When these are ingested by a mosquito, they develop into male and female gametes. A sequence of events now occurs in the mosquito gut and eventually leads to the release of sporozoites, which migrate to the salivary gland and are then capable of being transmitted through a bite to a new host.23
Black flies
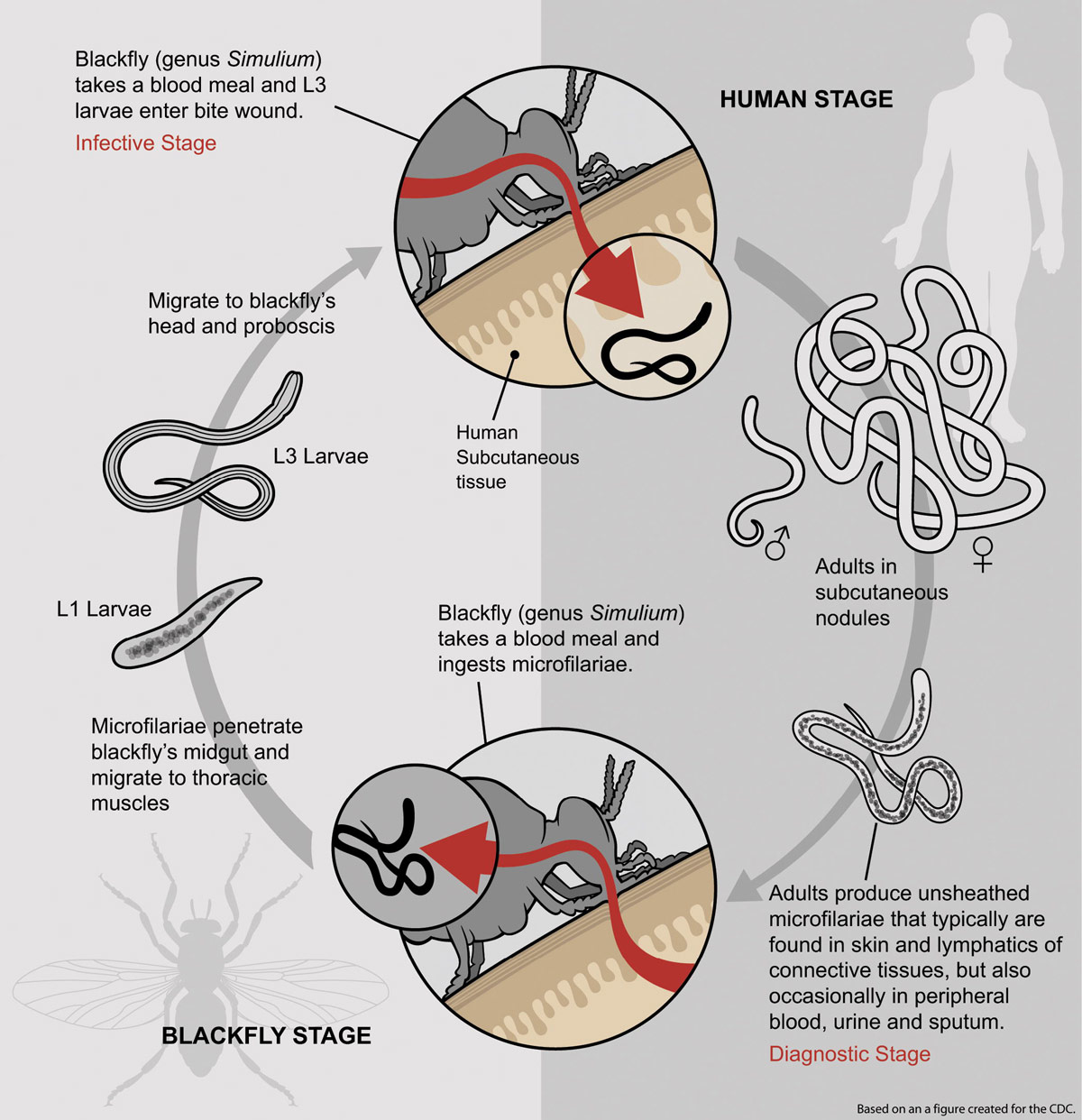
During an insect bite a third-stage filarial larva is introduced into the bloodstream (figure 3). The black flies transmit mature larvae to humans when the fly is taking a blood meal. These develop into adult filaria in subcutaneous connective tissue (nodules form). The female worms produce unsheathed small worms (microfilariae). These are found in selected body fluids including the blood. A progressive series of events occurs in the black fly before transmission in a blood meal can take place. The ingested microfilariae penetrate the black fly’s midgut and then migrate to the thoracic muscles and finally to the head and proboscis.24
Origin of disease organisms associated with blood-feeding insects
Whether the progenitors of the life forms we now observe are considered to have originated through naturalistic means or through creation, there are unresolved issues in generating reasonable schemes to explain the origin of disease organisms transmitted through blood-feeding insects.
Evolutionary views
Various theories exist among evolutionists on the likely route for the emergence of diseases associated with bloodsucking insects. The solutions proposed are based mainly on comparing genetic and other homologies among organisms and then devising routes of advance from the simple to more complex organisms.
Horizontal transfer of genes through endosymbiotic or other unknown processes is deemed responsible for the added complexity needed to explain the transition of life from primitive to complex forms. Naturally, through this process new genetic information is added to the recipient genome, which is essential if any theory of evolutionary progress is to gain credibility.
The gold standard used by evolutionists in such studies is to construct a phylogenetic tree of supposedly related species. When the genomes of these species are analyzed, horizontal gene transfer supposedly is indicated when a gene sequence(s) is identified in unrelated species. Even this method has acknowledged difficulties because in the first instance there is dispute over phylogenetic relationships. Other methods to detect gene transfer can also be used, but are considered less reliable than the gold standard. Sometimes supposed exchanges have been shown to be totally erroneous.25 Then, too, contamination is an ever present issue. The advice is to provide complete data on the immigrant sequence, integration sites, host genome flanking regions, a sensible transfer mechanism, and a detailed phylogenetic context. These safeguards may not be met. This means that false claims can be made easily.26 Added to these issues are that sampling biases affect interpretation of results even to the extent that instead of the data supporting horizontal gene transfer, vertical transfer and gene loss may be a more realistic possibility.27
The basic problem with this approach is that homology does not indicate kinship. It simply represents the fact that different organisms possess similar strategies to achieve related outcomes.
Malarial parasites
The route for the possible emergence of the malarial parasite has attracted considerable interest. Several of the leading ideas will be mentioned.
The malaria organism belongs to a group that contains a plastid (double membrane organelle) that has marked similarities to those possessed by photosynthetic algae, although in malarial species the plastids have lost their photosynthetic ability. Consistent with this loss of ability, many genes are missing. The suggested pathway leading to the transformation of non-pathogen to obligate pathogen remains obscure, but it has been noted that some of the genera related to the malaria organism are parasitic on marine invertebrates.28 Two genera of colpedellids (Chromera and Vitella) have been the focus of scientific attention. The chromerids are closely associated with corals.29
The colpodellids are a group of single-celled eukaryote organisms. They show a variety of interactions with other organisms including commensalism, predation, and parasitism (intracellular). They have a decisive role in the regulation of protists and algal communities in aquatic habitats. If these organisms were modified to give rise to the malaria organism, it has been suggested that horizontal gene transfer as well as gene loss would need to have been involved in the appearance of apicomplexan parasitism.30
A postulated place for such an event to take place is in the larval mosquito gut. An array of food is ingested, including algae, bacteria, diatoms, protozoa, rotifers, and crustaceans.31 Since the colpodellids are small (<20μm), possess even smaller cysts and have a motile zoospore stage,32 ingestion would not be an issue. However, no suggestions are made on how coral-associated organisms (marine) interacted with mosquito larvae (freshwater/brackish) or of the interactions occurring in salivary glands and the larval mosquito gut to help providence or gene transfer in the natural environment.
Other possibilities mentioned are that the malaria organism was modified from some of the blood parasites of frogs and snakes capable of being transmitted by ingesting mosquitoes.33
The life cycle of two Hepatazoon species found in frogs (Rana) have similarities to that shown by the malaria organism including the occurrence of asexual reproduction in frogs with merozoites invading erythrocytes and asexual reproduction in mosquitoes. Sporocysts are formed in the Malpighian tubules of the mosquito and represent the final stage of the sexual cycle. The organism is transmitted to frogs through ingestion of the mosquitoes. Asexual reproduction takes place in the liver of frogs using one round of merogony (1 to 2 rounds for Plasmodium). The merozoites produced invade erythrocytes and then are transformed into gamonts (sexual stage) through binary fission. Mosquitoes acquire the parasite by feeding on infected frogs.34 Adaptation of such parasites to the constant high temperature found in mammals is one issue these parasites would face.35 Nothing is known of the ability of Herpatazoon species to survive under such conditions. More fundamental issues, such as adaptation of the metabolism to a new host and the ability to avoid destruction by the immune system, would also be faced. Adaptation to a human host would present major challenges unless it was genetically programmed to cope with significantly different physiology including endothermy and unique features of the human immune response.
Such barriers may have been less formidable than imagined as it has been shown recently that, in experimental situations, mice could become infected by a Hepatazoon parasite of snakes (H. ayorgbor) and could in turn transmit the organism to snakes when consumed.36
Filarial parasites
Black flies are often infected with nematodes, bacteria, fungi, protozoa and viruses.37 It is known that there are some nematode pathogens of the fly larvae (Merminthids) and sometimes these parasites persist into the adults. They are free living as adults and have affinities to other nematodes parasitic as animal and plant pathogens.38 Further, some nematodes carried by black flies infect the vertebrates they feed on and cause disease.38
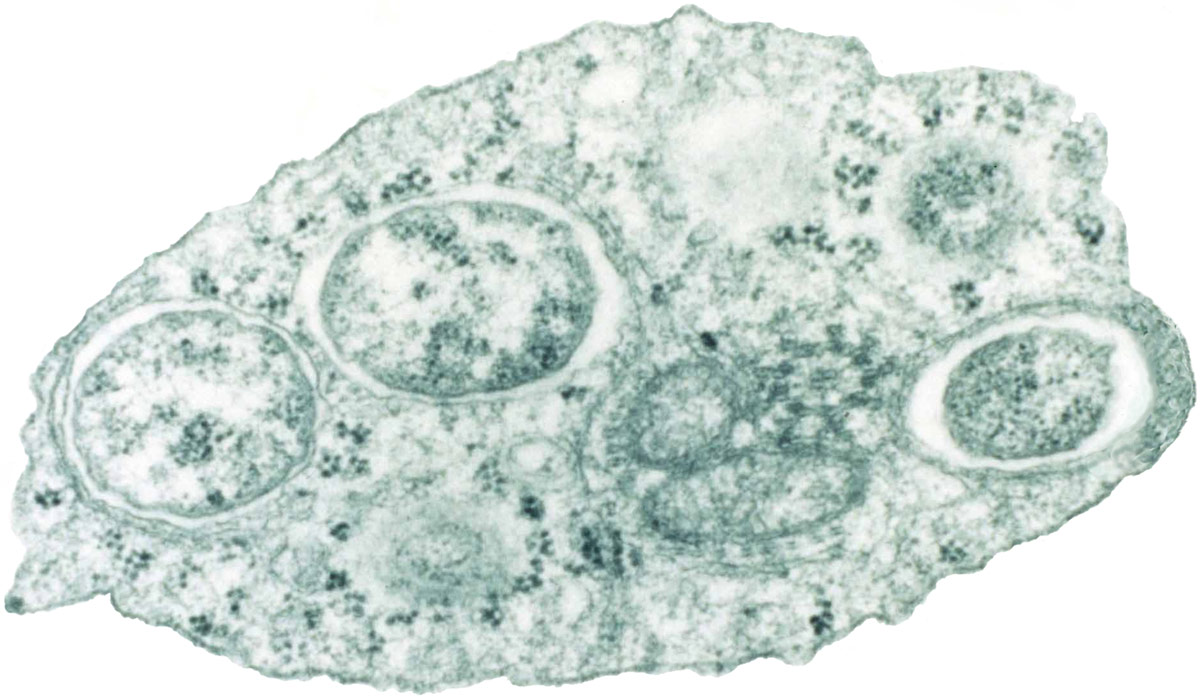
Such infection has been linked with data coming from the study of Wolbachia bacteria (figure 4), which many filarial parasites carry, including O. volvulus (river blindness nematode). The bacteria are transmitted to the next generation of insects via eggs. They appear to have a contributing role to the parasitic nature of the worms on other hosts or to interfere with the host immune response. Indeed, antibiotics can render the nematodes ineffective in causing disease, which establishes that Wolbachia performs a vital function in the nematode’s lifestyle.39
Gene transfer from the Wolbachia bacteria to arthropods and nematodes (eukaryotes) that carry them is touted as a distinct possibility in some circles, since these organisms are closely associated with germ-line cells. An intriguing example of putative transfer has been cited in the adzuki bean beetle (Callosobruchus chinensis). Genes in the X chromosome of the beetle are identical to those of the bacterium it carries.40 The Wolbachia bacteria, which are abundant and widespread in insects, do not have an independent existence, and also manipulate the insects’ reproductive biology.41
One problem with the African eye worm is that it does not carry the Wolbachia bacteria. However, remnants of gene sequences from the bacteria are thought to be present in the African eye worm filarial nematode and a related rodent parasite (Acanthocheilonema viteae).42 If this is so, these sequences or other changes wrought by the insertion of genetic material are thought potentially responsible for the pathogenic abilities now shown by the eye worm parasite.43
Creationist views—what changed immediately after the Fall?
It is speculative to suggest that insects with biting mouthparts used fruit and other plant tissues as their original food source and their move to imbibing blood may have developed after the Fall. However, an apparent adaptation for blood-feeding propensities has been observed in adult male fruit-feeding moths of Calyptra thalictri, which may give some strength to such an argument.44
Many taxa among the arthropods and nematodes form symbiotic relationships with prokaryotes. These symbiotic organisms can provide essential nutrients and other factors required for their reproduction and survival. The benefits may extend to providing defence against damaging organisms and limitation of stress from environmental factors. Not all of these bacteria may now confer benefits on the host. Those bacteria that have successful beneficial relationships with hosts possess smaller genomes than their free-living relatives.45
One reasonably can consider that beneficial (mutualistic) relationships among living organisms was the creation norm. This proposition is given strong credibility by the observation that a majority of plants form such relationships with microbes and other groups of living organisms have strong dependent relationships on unrelated groups.46
There is another body of thought among some creationists that death of organisms with no conscious feeling of pain occurred before the Fall (e.g. birds eating insects, phagocytic cells in the mammalian body disposing of bacteria entering the bloodstream). Some prefer to think that negative reproductive feedback mechanisms were in operation. Whenever disease organisms appeared, their emergence can be explained with varying degrees of success by invoking known phenomena facilitating gene transfer (transformation, transduction, and anastomosis), as has been shown elsewhere.47 Some of these and related phenomena have been shown to give rise to new pathogens in nature.
After the Fall, the design functions of the human body began to operate sub-optimally (Genesis 3:16—childbirth). This perhaps gives licence to suggest that malfunctions and mistakes would be noted in other life forms involving a range of processes. It could be expected from the above text that such changes would begin to appear in the short term.
Changes through time

For creationists, theories embracing insects predacious on others (entomophagous insects) and/or plant-sucking insects as the commencing point48 appear to be the ones likely to be most attractive. A significant point is that both insect groups, that are the focus of this article, have the ability to pierce surfaces and obtain nourishment from living tissues. This ability, we carefully note, is in life forms that do not possess conscious feeling of pain.
Mammalian biting ability of mosquitoes
Some suggestions are made relating to malaria that could appeal to creationists. Wyeomyia smithii is a species that lives as a commensal in the water-filled cavities of the purple pitcher plant (Sarracenia purpurea—figure 5).49 Biting, disinterested non-biting, and obligate non-biting populations of this mosquito have been identified. These populations are fully inter-fertile. Biting propensity in a low-biting population can be increased by selective manipulation.50 If the creation starting point was the obligate non-biting group of mosquitoes this ostensibly makes for a more comfortable argument. However, transitioning to a mammalian feeding habit would expose the mosquito to the problem of acquiring the ability to digest haem and contend with toxic metabolites. These may represent considerable hurdles. Further advances in explanation will be possible as scientists investigate the abilities held in both biting and non-biting insects.51
Not surprisingly, differences in gene expression have been identified among the three populations with the greatest difference seen between the biter and obligate non-biter population. The difference in differential gene expression between these two populations was five percent. The obligate non-biters were more flexible in their ability to adapt to changing environmental conditions. The biters, on the other hand, on account of partaking of a blood meal, sustain a cost in terms of metabolic activity, such as the need to breakdown hemoglobin, dispose of the toxins released, and to emphasize olfactory rather than visual inputs in seeking a food source. Some of the differences could be accounted for by changes in gene expression levels. In contrast to the proposal with which creationists would be comfortable, the supposed evolutionary sequence is from biter to obligate non-biter.52
If the salivary gland enzymes are considered in general, the most significant enzyme components identified in a number of blood-feeding insects are serine proteases and their inhibitors, hyaluronidases and apyrase. Now, similar enzymes are found in the plant kingdom or among some bacteria. No one has provided a convincing explanation how blood-feeders acquired these enzymes.17 Is it possible that the appropriate genes and small amounts of the key enzymes were present in the insect population, with selection and increased gene expression taking place after the Fall? Alternatively, or in addition, there may have been a microbe assisted transfer of phage carrying regulatory factors capable of increasing gene expression.
It has been noted that hyaluronidase activity can be phage encoded or associated with the phage particle, as in streptococci.53 It also has been observed that the mosquito genome contains plant-like sequences. It has been postulated that these may have been acquired as a consequence of the plant-feeding habits of the insect with input from the unique microbiota present in its salivary gland and midgut.54 This may be conceptually possible perhaps after the fashion of events noted in a Chlorella-like green alga. When this alga was infected with a virus carrying the hyaluronan synthase gene, the alga was able to synthesize the gene product within 15–30 minutes of infection. The representative polysaccharide (hyaluronic acid) appeared on the outside of its cell wall.55
Malarial parasite origin
The origin of the malarial parasite remains a mystery but the best possibility seems to reside with a group of apicomplexan organisms found in frogs. It is possible that parasites related to the present genus Hepatazoon were creation companions of frogs and exerting no adverse effects upon the creatures. This situation may have changed after the Fall. Today, Hepatazoon species are found in frogs and show many similarities to the life cycle now displayed by the malaria organism and furthermore they can be transmitted by mosquitoes, as mentioned in the last section. The suggestion made here is that originally these parasites lived in a balanced relationship with the host causing no adverse effects and perhaps even conferring some benefits. In some animals, such a postulated relationship may show similarities to those observed currently.56
Altering the complex interactions between parasites and their hosts and among microbes in a host may have dramatic consequences. In mosquitoes, modifying the immune response of the insect by gene manipulation of a transcription factor renders it almost completely resistant to malaria parasite transmission,57 which illustrates the effect of small changes in gene structure on outcomes.
Mosquito species of primary significance in hosting the malaria parasites do not generally carry Wolbachia bacteria. Their ability to do so can be significantly reduced if the bacterium is artificially introduced, as can their ability to transmit two virus disease entities.58 How a postulated loss of Wolbachia, a change in a key transcription factor, or other related changes might have occurred is at present not known.
Black flies and filarial parasites
A satisfactory explanation for the blood appetites of black flies and their ability to transmit filarial parasites is more difficult to explain than in mosquitoes with malaria. It has been noted that not all black flies with well-developed mandibles bite.59 The nutrients in blood theoretically are capable of being accessed from plant sources.21
Could it have been possible that the filarial parasites arose from the Merminthid nematode parasites of black flies with the aid of bacterial symbionts? Clearly, the process would have been an extended one for first the disease-causing nematode parasites of the black flies need to be explained. This could potentially take the line that some nematodes were designed to regulate insect populations. There are well-known examples of such regulation. Some of these nematodes are associated with bacterial symbionts that assist them in their controlling activities.60
Manipulation of gene expression through the microbiota in insects
Is it possible that the DNA of endosymbiotic bacteria may have been incorporated into the eukaryote nucleus of the host and changed its function? In order to answer this question, several examples can be mentioned. For example, the pea aphid (Acyrthosiphon pisum) requires the presence of a symbiont bacterium (Buchnera) to function and the bacterium also requires the aphid. At stages of host development the bacterium and germ line cells are not separated by membranes. Certain genes carried by the aphid are similar to prokaryote genes, and these are essential for viability. Where these genes originated from is a baffling question as it seems they are similar to those of bacteria that are present only sporadically in these insects. This pattern occurs in other insects studied—the DNA does not come from the symbiotic bacteria usually present.61 Indeed, it is acknowledged that many prokaryote-type genes appear in eukaryotes.62 At the moment, there are enormous gaps in our knowledge, leaving us to indicate again that gene similarity in nature does not necessarily indicate the original source of derivation of the gene or whether the “homologous” gene was created independently in several taxa.
Altering the complex interactions between parasites and their hosts and among microbes in a host may have dramatic consequences. The immune response of mosquitoes can be modified by transcription factor manipulation so that it is almost completely resistant to malaria parasite transmission.63 This represents an area deserving more extensive study. As already mentioned, mosquito species hosting the malarial parasites do not generally carry Wolbachia bacteria. Their vectoring ability can be significantly reduced if the bacterium is artificially introduced.58 Is it possible that a postulated loss of Wolbachia was a key event in the emergence of malaria in its present form?
Concluding remarks
Accounting for the presence of destructive parasites is a challenging exercise. Some progress has been made in this direction with mosquitoes and black flies. With both these groups it is suggested that plant and even invertebrate feeding occurred originally. These insects now would have had expanded food gathering possibilities, ostensibly acquired as ecological conditions changed and food resources became scarcer and less nourishing as a result of the Curse. Alternatively, dispersal to other localities may have occurred giving greater food gathering opportunities. It is possible that adaptive processes could account for many of these changes. This could include expression of front-loaded genetic material or information coming from elsewhere as a result of microbial assisted transfer. Currently, insufficient detail is known about the insects, their salivary enzymes, changing conditions, the parasites they vector, associated bacteria affecting these relationships, horizontal gene transfer, and other unknowns, to make any conclusive remarks.
The origin of malaria parasite and the river blindness parasite carried by insects also poses problems. However, the problems are much worse for those approaching the problem from a naturalistic evolutionary perspective. Parasitic nematodes capable of controlling insect populations are a possible source from which the river blindness parasite arose. Such an event would have involved an ecological niche change for the vector and the parasite, conceivably involved gene transfer acquisition and/or loss through known mechanisms. Mosquitoes are hosts to nematodes, fungi, bacteria, protists, viruses, and other microscopic organisms. The similarity of the malaria parasite to mosquito-transmitted organisms in frogs suggests a possible origin. The postulated loss of a mosquito-associated bacterium may have assisted in the ability of the parasite to develop in its present form.
The proposed wide movements of genetic information across recognized genera by evolutionists often cannot be accomplished in the laboratory and in many instances are not accompanied by reasonable suggestions of mechanisms that would facilitate such exchange in the natural world. This means that many of the ancestral schemes constructed are without a sound foundation. In all instances where genetic exchange has been demonstrated experimentally, the recipient organism is still recognizable. It may have acquired some new abilities or lost some capabilities, but its basic structural and functional features are recognizable as belonging to a particular group of organisms.
References and notes
- Buchanan, M, Wild, Wild Life, Sydney Morning Herald, The Guide, p. 6, 24 March 2003; quoted in McIntosh, A. and Hodge B., How did defense/attack structures come about? in: Ham, K. (Ed.), The New Answers Book, Master Books, Green Forest, AR, 1:259–270, 2012. Return to text.
- World Health Organization, Malaria, 2016, www.who.int/mediacentre/factsheets/fs094/en/ Return to text.
- Cardé, R.T., Mulit-cue integration: how female mosquitoes locate a human host, Current Biology 25(18):R793–R795, 2015 | doi:10.1016/j.cub.2015.07.057. Return to text.
- George, J., Blanford. S., Thomas, M. B., and Baker, T.C., Malaria mosquitoes host-locate and feed upon caterpillars, PLoS ONE 9(11):e108894, 2014 | doi:10.1371/journal.pone.0108894. Return to text.
- Sutcliffe, J.F., Steer, D.J., and Beardsall, D., Studies of host location behaviour in the black fly Simulium arcticum (IIS-1O.11) (Diptera: Simuliidae): aspects of close range trap orientation, Bulletin of Entomological Research 85(3):415–424, 1995. Return to text.
- Gullan, P.J. and Cranston, P.S., The Insects: An outline of entomology, 3rd edn, Blackwell Publishing Ltd., Malden, MA, 2005. Return to text.
- World Health Organization, Mosquito ecology, 1967, apps.who.int/iris/handle/10665/40662. Return to text.
- Calvo, E., Pham, V.M., and Ribeiro, J.M., An insight into the sialotranscriptome of the non-blood feeding Toxorhynchites amboinensis mosquito, Insect Biochemistry and Molecular Biology 38(5):499–507, 2008 | doi:10.1016/j.ibmb.2007.12.006; Hien, D.F. d.S., Dabiré, K.R., Roche, B. et al., Plantmediated effects on mosquito capacity to transmit human malaria, PLoS Pathogens 12(8):e1005773 | doi:10.1371/journal.ppat.1005773; Foster, W.A., Mosquito sugar feeding and reproduction energetics, Annual Review of Entomology 40:443–474, 1995; Rattanarithikul, R., Harbach, R., Harrison, B.A., Panthusiri, P., and Coleman, R.E., Illustrated keys to the mosquitoes of Thailand, V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. Southeast Asian Journal of Tropical Medicine and Public Health 2:1–65, 2007; Spielman, A., Bionomics of autogenous mosquitoes, Annual Review of Entomology 16:231–248, 1971. Return to text.
- Harris, P. and Cooke, D., Survival and fecundity of mosquitoes fed on insect haemolymph, Nature 222(5200):1264–1265, 1969. Return to text.
- Spielman, ref. 8. Return to text.
- McCreadie, J.W., Adler, P.H., and Beard, C.E., Ecology of symbiotes of larval black flies (Diptera: Simuliidae): distribution, diversity, and scale, Environmental Entomology 40(2):289–302, 2011 | doi:10.1603/EN10258; Pennsylvania Government, Department of Environmental Protection, Black fly–Biology, 2019, dep.pa.gov/Business/Water/CleanWater/BlackFly/Pages/Biology.aspx. Return to text.
- McKeever, S. and French, F E., Fascinating beautiful blood feeders: deer and horse flies, the Tabanidae, American Entomologist, Winter, 217–226, 1997; Steel, B., Chrysops excitans, 2014, Animal Diversity Web, animaldiversity.org/accounts/Chrysops_excitans/. Return to text.
- Hunter, B., Rohner, C., and Currie, D.C., Black-flies and Leucocytozoan spp. as causes of mortality in juvenile giant horned owls in the Yukon, Canada; in: Duncan, J.R., Johnson, D. H., and Nicholls, T.H. (Eds), Biology and Conservation of Owls of the Northern Hemisphere, 2nd International Symposium, General Technical Report NC-190, US Department of Agriculture, Forest Service, North Central Forest Experiment Station, St Paul, MN, pp. 243–245, 1997. Return to text.
- Burgin, S.G. and Hunter, F.F., Nectar versus honeydew as sources of sugar for male and female black flies (Diptera: Simuliidae), J. Medical Entomology 34(6):605–608, 1997 | doi: 10.1093/jmedent/34.6.605; Ossowski, A. and Hunter, F.F., Distribution patterns, body size, and sugar-feeding habits of two species of Chrysops (Diptera: Tabanidae), Canadian Entomologist 132(2):213–221, 2000. Return to text.
- Calvo et al., ref. 8; Foster, ref. 8; Spielman, ref. 8. Return to text.
- Mans, B.J., Evolution of vertebrate hemostatic and inflammatory control mechanisms in blood-feeding arthropods, J. Innate Immunity 3(1):41–51, 2011. Return to text.
- Chagas, A.C., Calvo, E., Pimenta, F.F.P. et al., An insight into the sialome of Simulium guianense (Diptera: Simuliidae), the main vector of River Blindness Disease in Brazil, BMC Genomics 12:612, 2011 | doi:10.1186/1471-2164-12-612. Return to text.
- Wu, Q. and Brown, M. R., Signaling and function of insulin-like peptides in insects, Annual Review of Entomology 51:1–24, 2006. Return to text.
- Ohtaki, T., Milkman, R.D., and Williams, C.M., Ecdysone and ecdysone analogues: their assay on fleshfly Sarcophaga peregrine, PNAS 58(3):981–984, 1967; Spielman, A., Gwadz, R.W., and Anderson, W.A., Ecdysone-initiated ovarian development in mosquitoes, J. Insect Physiology 17(10):1807–1814, 1971. Return to text.
- Noriega, R., Ramberg, F.B., and Hagedorn, H.H., Ecdysteroids and oocyte development in the black fly Simulium vittatum, BMC Developmental Biology 2:6, 2002 | doi:10.1186/1471-213X-2-6. Return to text.
- Adler, J.H. and Grebenok, R.J., Occurrence, biosynthesis, and putative role of ecdysteroids in plants, Critical Reviews in Biochemistry and Molecular Biology 34(4):253–264, 1999. Return to text.
- Nation, J.L., Insect Physiology and Biochemistry, 2nd edn, CRC Press, Boca Raton, FL, pp. 135–136, 2008. Return to text.
- Willey, J.M., Sherwood, L.M., and Woolverton, C.J., Prescott’s Microbiology, 8th edn, McGraw-Hill International, New York, p. 332, 2011. Return to text.
- Centers for Disease Control and Prevention, Onchocerciasis, 2017, cdc.gov/dpdx/onchocerciasis/index.html. Return to text.
- Stanhope, M.J., Lupas, A., Italia, M.J. et al., Phylogenetic analysis do not support horizontal gene transfers from bacteria to vertebrates, Nature 441:940–944, 2001. Return to text.
- Wijayawardena, B.K., Minchella, D.J., and DeWoody, J.A., Host, parasites, and horizontal gene transfer, Trends in Parasitology 29(7):329–338, 2015. Return to text.
- Gluck-Thaler, E. and Slot, J.C., Dimensions of horizontal gene transfer in eukaryotic microbial pathogens, PLoS Pathogens 11(10):e1005156, 2015 | doi:10.1371/journal.ppat.1005156. Return to text.
- Janouškovec, J., Horák, A., Oborník, M. et al., A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids, PNAS 107(24):10949– 10954, 2010; Woo, Y.H., Ansari, H., Thomas, O.D. et al., Chromerid genomes reveal the evolutionary path from photosynthetic algae to obligate intracellular parasites, Genomics and Evolutionary Biology 4:e06974, 2015 | doi:10.7554/eLife.06974. Return to text.
- Moore, R.B., Oborník, M., Janouškovec, J. et al., A photosynthetic alveolate closely related to apicomplexan parasites, Nature 451:959-963, 2008; Woo et al., ref. 28. Return to text.
- Brugerolle, G., Cryptophagus subtilis: a new parasite of cryptophytes affiliated with the Perkinozoa lineage, European J. Protozoology 37(4):379–390, 2002; Brugerolle, G., Colpodella vorax: ultrastructure, predation, lifecycle, mitosis, and phylogenetic relationships, European J. Protistology 38(2):113–125, 2002; Templeton, T.J. and Pain, A., Diversity of extracellular proteins during the transition from the ‘proto-apicomplexan’ alveolates to the apicomplexan obligate parasites, Parasitology 143(1):1–17, 2016 | doi:10.1017/S0031182015001213;Yuan, C.L., Keeling, P.J., Krause, P.J. et al., Colpodella spp.–like parasite infection in woman, China, Emerging Infectious Diseases 18(1):125–127, 2012 | doi:10.3201/eid1801.110716. Return to text.
- Merritt, R.W., Dadd, R.H., and Walker, E.D., Feeding behaviour, natural food, and nutritional relationships of larval mosquitoes, Annual Review of Entomology 37:349–376. 1992. Return to text.
- Leander, B.S., Kuvardina, O.N., Aleshin, V.V. et al., Molecular phylogeny and surface morphology of Colpodella edax (Alveolata): insights into the phagocytic ancestry of apicomplexans, J. Eukaryotic Microbiology 50(5):334–340, 2003. Return to text.
- Barta, J.R., Ogedengbe, J.D., Martin, D.S. et al., Phylogenetic position of the adeleorinid coccidia (Myzozoa, Apicomplexa, Coccidia, Eucoccidiorida, Adeleorina) inferred using 18A rDNA, J. Eurkaryotic Microbiology 59(2):171–180, 2012; Smith, T.G., Kim, B., Hong, H. et al., Intraerythrocytic development of species of Hepatozoon infecting ranid frogs: evidence for convergence of life cycle characteristics among apicomplexans, J. Parasitology 86(3):451–458, 2000. Return to text.
- Desser, S., Hong, H., and Martin, D.S., The life history, ultrastructure, and experimental transmission of Hepatozoon catesbianae n. comb., an apicomplexan parasite of the bullfrog, Rana catesbeina and the mosquito, Culex territans in Algonquin Park, Ontario, J. Parasitology 81(2):212–222, 1995; Ferguson, L.V., Hillier, N.K., and Smith, T.G., Influence of Hepatazoon parasites on host-seeking and host-choice behaviour of the mosquitoes Culex territans and Culex pipiens, International J. Parasitology: Parasites and Wildlife 2:69–76, 2013; Leander, B.S., Lloyd, S.A.J., Marshall, W. et al., Phylogeny of marine gregarines (Apicomplexa)–Pterospora, Lithocystis and Lankesteria–and the origin(s) of coelomic parasitism, Protist 157(1):45–60, 2006; Maxwell, R.D., Gregarines and Haemogregarines; in: Kreir, J.P. (Ed.), Gregarines, Haemogregarines, Coccidia, Plasmodia, and Haemoproteids, Academic Press Inc., London, pp. 1–32, 1977. Return to text.
- Jancarova, M., Hlavacova, J., Votypka, J. et al., An increase of larval rearing temperature does not affect the susceptibility of Phlebotomus sergenti to Leishmania tropica but effectively eliminates the gregarine Psychodiella sergenti, Parasites and Vectors 9:553, 2016 | doi: 10.1186/s13071-016-1841-6. Return to text.
- Sloboda, M., Kamler, M., Bulantova, J. et al., Rodents as intermediate hosts of Hepatozoon ayorgbor (Apicomplexa: Adeleina: Hepatozoidae) from the African ball python, Python regius, Folia Parasitologica 55:13–16, 2008. Return to text.
- Pennsylvania Government, ref. 11. Return to text.
- De Ley, P., A quick tour of nematode diversity and backbone of nematode phylogeny, WormBook, 2006, wormbook.org/chapters/www_quicktourdiversity/quicktourdiversity.html; McCreadie et al., ref. 11. Return to text.
- Ferri, E., Bain, O., Barbuto, M. et al., New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species, PLoS One 6(6):e20843, 2011 | doi:10.1371/journal.pone.0020843; Wu, B., Novelli, J., Jiang, D. et al., Interdomain lateral gene transfer of an essential ferrochelatase gene in human parasitic nematodes, PNAS 110(19):7748–7753, 2013. Return to text.
- Nikoh, N., Tanaka, K., Shibata, F. et al., Wolbachia genome integrated in an insect chromosome: evolution and fate of laterally transferred endosymbiont genes, Genome Research 18(2):272–280, 2008. Return to text.
- Bouchery, T., Lefoulon, E., Karadjian, G. et al., The symbiotic role of Wolbachia in Onchocercidae and its impact on filariasis, Annual Review of Ecology and Systematics 32:519–545, 2001; Werren, J.H., Biology of Wolbachia, Annual Review of Entomology 42:587–609, 1997. Return to text.
- Ferri et al., ref. 40; Desjardins, C.A., Cerqueira, G.C., Goldberg, J.M. et al., Genomics of Loa, a Wolbachia-free filarial parasite of humans, Nature Genetics 45(5):495–500, 2013 | doi:10.1038/ng.2585; McNully, S.N., Foster, J.M., Mitreva, M. et al., Endosymbiont DNA in endobacteria-free filarial nematodes indicates ancient horizontal genetic transfer, PLoS One 5(6):e11029, 2010 | doi:10.1371/journal.pone.0011029. Return to text.
- Scott, A.L., Ghedin, E., Nutman, T.B. et al., Filarial and Wolbachia genomics, Parasite Immunology 34(2–3):121–129, 2012. Return to text.
- Hill, S.R., Zaspel, J., Weller, S., Hansson, B.S., and Ignell, R., To be or not to be… a vampire: a matter of sensillum numbers in Calyptra thalictri? Arthropod Structure and Development 39(5):322–333, 2010. Return to text.
- Moran, N.A. and Bennett, G.M., The tiniest tiny genomes, Annual Review of Microbiology 68:195–215, 2014; Moya, A., Peretó, J., Gil, R. et al., Learning how to live together: genomic insights into prokaryote-animal symbioses, Nature Reviews Genetics 9:218–229, 2008. Return to text.
- Brundrett, M.C., Coevolution of roots and mycorrhizas of land plants, New Phytologist 154(2):275–304, 2002; Hamel, C., Impact of arbuscular mycorrhizal fungi on N and P cycling in the root zone, Canadian J. Soil Science 84(4):355–363, 2004. Return to text.
- Shipton, W.A., Origins of pathogenic microbes: part 1—bacteria, J. Creation 30(2):76–82, 2016; Shipton, W., Accounting for blighting plant and disfiguring animal diseases, J. Creation 32(3):97–104, 2018. Return to text.
- de Silva, P. and Bernal, X., Evolution of blood sucking insects, 2013, academia.edu/6446149/Evolution_of_blood_sucking_insects; Lehane, M.J., The Biology of Blood-Sucking in Insects, 2nd edn, Cambridge University Press, Cambridge, p. 14, 2005. Return to text.
- Peterson, C.N., Day, S., Wolfe, B.E., Ellison, A.M. et al., A keystone predator controls bacterial diversity in the pitcher-plant (Sarracenia purpurea) microecosystem, Environmental Microbiology 10(9):2257–2266, 2008 | doi:10.1111/j.1462-2920.2008.01648.x. Return to text.
- Spielman, ref. 8. Return to text.
- Whiten, S.R., Eggleston, H., and Adelman, Z.N., Ironing out the details: exploring the role of iron and heme in blood-sucking arthropods, Frontiers in Physiology, 17 January 2018, frontiersin.org/articles/10.3389/fphys.2017.01134/full. Return to text.
- Bradshaw, W.E., Burkhart, J., Colbourne, J.K. et al., Evolutionary transition from blood feeding to obligate nonbiting in a mosquito, PNAS 115(5):1009–1014, 2018. Return to text.
- Wagner, P.L. and Waldor, M.K., Bacteriophage control of bacterial virulence, Infection and Immunity 70(8):3985–3993, 2002. Return to text.
- Sharma, P., Das De, T., Sharma, S. et al., Deep sequencing revealed molecular signature of horizontal gene transfer of plant like transcripts in mosquito Anopheles culicifacies: an evolutionary puzzle, F1000 2015 4:1523, 2015 | doi:10.12688/f1000research.7534.1. Return to text.
- Graves, M.V., Burbank, D.E., Roth, R. et al., Hyaluronan synthesis in virus PBCV-1 infected Chorella-like green algae, Virology 257(1):15–23, 1999. Return to text.
- Maia, J.P., Harris, D.J., Carranza, S., and Gómez-Díaz, E., A comparison of multiple methods for estimating parasitemia of hemogregarine hemoparasites (Apicomplexa: Adeleorina) and its application for studying infection in natural populations, PLoS ONE 9(4):e95010, year | doi:10.1371/journal.pone.0095010; Sailasuta, A., Satetasit, J., and Chutmongkonkul, M., Pathological study of blood parasites in rice field frogs, Hoplobatrachus rugulosus (Wiegmann, 1834), Veterinary Medicine International 2011 | doi:10.4061/2011/850568. Return to text.
- Dong, Y., Das, S., Cirimotich, C. et al., Engineered Anopheles immunity to Plasmodium infection, PLoS Pathogens 7(12):e1002458 | doi:10.1371/journal.ppat.1002458. Return to text.
- Baldini, F., Segata, N., Pompon, J. et al., Evidence of natural Wolbachia infections in field populations of Anopheles gambiae, Nature Communications 5:3985, 2014 | doi:10.1038/ncomms4985; Moreira, L.A., Iturbe-Omaetxe, I., Jeffery, J.A. et al., A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium, Cell 139(7):1268–1278, 2009. Return to text.
- Mckeever and French, ref. 12. Return to text.
- Chaston, J. and Goodrich-Blair, H., Common trends in mutualism revealed by model associations between invertebrates and bacteria, FEMS Microbiology Reviews 34(1):41–58, 2010. Return to text.
- Nikoh, N. and Nakabachi, A., Aphids acquired symbiotic genes via lateral gene transfer, BMC Biology 7:12, 2009; Moran and Bennett, ref. 45. Return to text.
- Doolittle, W.F., You are what you eat: a gene transfer ratchet could account for bacterial genes in eukaryotic nuclear genomes, Trends in Genetics 14(8):307–311, 1998. Return to text.
- Dong, Y., Das, S., Cirimotich, C. et al., Engineered Anopheles immunity to Plasmodium infection, PLoS Pathogens 7(12):e1002458 | doi:10.1371/journal.ppat.1002458 Return to text.





Readers’ comments
Comments are automatically closed 14 days after publication.