Did God create life?
Ask a protein
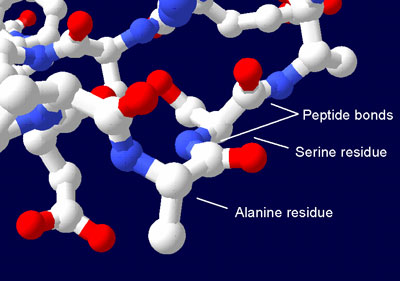
Most highschool students are taught that life began when lightning passing through a particular atmosphere produced chemicals called amino acids. These are the building blocks of proteins, the major ingredient of cells. In 1953, Stanley Miller’s famous experiment showed that some amino acids really can be produced in this way.
However, it’s one thing to get the building blocks, but quite another to get them to build. Supposedly, these amino acids went on to concentrate in the ocean in an organic broth where they linked together to form proteins. These proteins then somehow got together with DNA to form the first simple cell, or so it is said. Many who believe that life started without a Creator were at first convinced by this argument. Now even the atheists are bailing out. Why?
- Amino acids do not concentrate in the ocean. They disperse.
- Amino acids would be grossly contaminated with other chemicals that would stop them forming proteins.
- And even pure amino acids (made by intelligent organic chemists) will not form proteins under natural conditions. Rather, the reverse happens—proteins break down into amino acids.1
- Miller’s amino acids were an equal amount of ‘right-handed’ and ‘left-handed’ amino acids. Living things use exclusively left-handed ones.2
- Even if pure left-handed amino acids could link up, it could not be in the right order.3 In living things, this is coded in the DNA and read by complex machinery—requiring already-existing proteins!4
- DNA and its building blocks, called nucleotides, do not form spontaneously either.5
The argument that convinced multitudes that life did not need a Creator was false in every step except the first one; some amino acids can form in nature. A quiet revolution has taken place in the last few years. Another chemical has elbowed out proteins and taken over the popular fancy. Even schoolbooks are finally admitting that proteins could not have formed in organic broth:
‘Scientists have not been able to cause amino acids dissolved in water to join together to form proteins. The energy-requiring chemical reactions that join amino acids are freely reversible and do not occur spontaneously in water. However, most scientists no longer argue that the first proteins assembled spontaneously. Instead, they now propose that the initial macromolecules were composed of RNA, and that RNA later catalyzed the formation of proteins.’6
The stories have changed, but the central dogma, ‘Life did not require an intelligent Creator’, has remained the same. But the new proposal, ‘the initial macromolecules were composed of RNA, and that RNA later catalyzed the formation of proteins’, is false. RNA, like DNA, will not form outside of already living cells!7,8,9
Whatever one believes about their origin, proteins are the principle ingredients of living cells, and deserve serious consideration. Most people have no idea of the powerful scientific evidence they give that living things had an intelligent Creator.
Proteins are folded to fit
In order to perform its function in a cell, each protein must be folded correctly in its own complex three-dimensional shape. When a cell makes a new protein, on the way to its place in the cell, it folds into the exact shape which will allow it to connect with the other proteins or sugars, etc. It’s a bit like the way a key fits in a lock.
IBM has built the world’s most powerful supercomputer (dubbed Blue Gene, completed in 2005) to tackle the protein folding problem. The IBM website explains why:
‘The scientific community considers protein folding one of the most significant “grand challenges”—a fundamental problem in science … whose solution can be advanced only by applying high-performance computing technologies.
‘Proteins control almost all cellular processes in the human body. Comprising strings of amino acids that are joined like links of a chain, a protein folds into a highly complex, three-dimensional shape that determines its function. Any change in shape dramatically alters the function of a protein, and even the slightest change in the folding process can turn a desirable protein into a disease.’10
In spite of the tremendous amount of computing power being unleashed, it was estimated that it would still take about a year for Blue Gene to finish its calculations and model the folding of a simple protein. How long does it take living cells to actually fold one? Less than a second!
As one IBM researcher had earlier noted, ‘It’s absolutely amazing, the complexity of the problem and the simplicity with which the body does it every day.’11
Chaperones
Specialized proteins called chaperones or chaperonins have been discovered to be vital for folding many proteins. They move along with newly made proteins to the places in the cell, where they must fit perfectly if they are to function with the other proteins around them. On the way, the chaperones help them fold correctly, and then help fit them into their place. How do the chaperones themselves fold correctly? They too have chaperones! Evolutionists thus have another problem: how did the first chaperones ever fold correctly without pre-existing chaperones?12
Scientists are able to link amino acids in the laboratory to assemble some small proteins, but unless they fold properly they will not work in living things. Unfolded proteins may be the same chemically, but they are no better than miniature spaghetti as far as biological activity is concerned, and a wrong fold may cause a serious disease. One example is the deadly Creutzfeldt–Jakob disease (CJD) in humans, related to ‘mad cow’ disease.
Addressing proteins
Even though there may be billions of possible wrong places for some proteins to go, there are very few places, sometimes just one, in which any newly made protein will fit and function. The problem is that proteins are not made where they will be used, and each one is worthless until it has found its way to the spot where it fits. How do proteins find their way?
The answer is, ‘ … newly minted proteins contain an amino acid string that determines their eventual home.’13 This string of amino acids is usually added as a tail on the end of the longer string of amino acids which make up the protein. It has been compared to the address on an envelope. When you put a letter in the mail box without the address, what chance does it have of getting to the right person? Each properly folded protein will fit and connect correctly in only one spot, so it must be addressed correctly. ‘Misplacing a protein is more serious than losing a letter, however. “There are diseases where proteins are mistargeted in cells.”’13
In 1999, The Nobel Prize for Medicine went to Dr Guenter Blobel of The Rockefeller University in New York, for discovering the amino acid address tags that direct each protein to its proper place in the cell.14
For the first cell to function, it not only had to have a way to make proteins, it also had to have solved the complex problems of folding proteins correctly, and addressing them to the exact spots where they would fit and function. Near misses in any step can cause disease.
Turning proteins on and off
It is not enough for a cell’s proteins to be folded correctly and sent to the right places. The cell also needs the right amount of each protein. If it just kept making more and more copies of any given protein, it would use up many of its raw materials. It is like the difference between burning the right amount of wood in your fireplace, and burning down the whole house.
Also, if there were even one protein that the cell could not stop making after it had made enough, that cell would soon be jammed so full of that protein that it would ‘pop’. The production of every individual protein, therefore, must be turned on and off at just the right moment.15
Even if a first cell had just turned up with all the right amounts of the correct proteins, perfectly folded and in the right place to begin life, it would have had to replace each protein as soon as it wore out.
One of the most important methods of turning protein production on or off is regulatory DNA sequences. They are stretches of DNA whose job is to tell the cell when to start and stop the production of the various proteins. The DNA, however, cannot turn protein production on or off by itself. It works together with specialized proteins, each of which fits a particular stretch of regulatory DNA. The regulatory protein folds perfectly so it will fit the exact spot on the DNA with which it must work. Together they form a switch.16 Neither the regulatory DNA sequences nor the regulatory proteins will work without the other. Both must have come into being perfectly coordinated by the time production of the first protein needed to be turned on or off.
Proteins are so complex they will not form anywhere in nature except in living cells. Inside cells, the directions for protein construction are already contained in the DNA. Then, if a protein is to perform its task, its production must be carefully regulated, but even then, it will not function unless it also has the correct address tag and is properly folded. All these systems would have to have been in place or the ‘first cell’ could not function. These systems, however, are just the tip of the iceberg. I chose them to illustrate the many coordinated systems that would have to have been present before the first cell would work.
The teaching that the first cell spontaneously popped into being without the involvement of the Creator has its basis in the pre-scientific myth that single-celled creatures were simple. It obviously does not stand up under today’s knowledge that a cell’s DNA, RNA, membranes and proteins are extremely hard to make, and when proteins are made, they must be properly folded, addressed, and turned on and off at just the right times. None of these brilliant solutions could invent itself, yet no ‘first cell’ could exist without all of them. They could not have happened without a very intelligent Creator.
God’s solutions to these complex problems were, in fact, incomparably better than those hoped for from the world’s most powerful super computer. They remind us of how powerful and intelligent the Creator is. It’s only reasonable to trust Him with our lives.
References and notes
- Sarfati, J., Origin of life: the polymerization problem, Journal of Creation 12(3):281–284, 1998. Return to text.
- Sarfati, J., Origin of life: the chirality problem, Journal of Creation 12(3):263–266, 1998. Return to text.
- Grigg, R., Could monkeys type the 23rd Psalm? Creation13(1):30–34, 1990. Return to text.
- Sarfati, J. Self-replicating Enzymes? A critique of some current evolutionary origin-of-life models, Journal of Creation 11(1):4–6, 1997. Return to text.
- Sarfati, J., Origin of life: Instability of building blocks, Journal of Creation 13(2):124–127, 1999. Return to text.
- Johnson, G.B. and Raven, P.H., Biology, Principles & Explorations, Holt, Reinhart and Winston, Florida, USA, p. 235, 1998. Return to text.
- Fry, I., The Emergence of Life on Earth, Rutgers University Press, New Jersey, USA, pp. 126, 176–177, 245, 2000. Return to text.
- Ward, P.D. and Brownlee, D., Rare Earth, Why complex Life is Uncommon in the Universe, Copernicus, Rutgers University Press, New Jersey, USA, p. 65, see also pp. xix, 60, 63–64, 1999. Return to text.
- Mills, G.C. and Kenyon, D., The RNA World: A Critique, Origins & Design 17(1):9–16, 1996. Return to text.
- IBM and Department of Energy’s NNSA partner to expand IBM’s Blue Gene Research Project, www.research.ibm.com/bluegene/press_release.html, 28 November 2003. Return to text.
- Lohr, S., IBM plans a supercomputer that works at the speed of life, New York Times, 6 December, 1999, p. C1. Return to text.
- Aw, S.E., The Origin of Life: A Critique of Current Scientific Models, Journal of Creation 10(3):300–314, 1996. Return to text.
- Travis, J., Zip Code plan for proteins wins Nobel, Science News 156(16):246, 1999. Quote by Tom A. Rapoport of Harvard Medical School in Boston. See also Britannica Biography Collection, Guenter Blobel. Return to text.
- Cell Biologist Dr. Gunter Blobel, Nobel Laureate on how cells work, http://findarticles.com/p/articles/mi_hb155/is_1_17/ai_n28885273/, Accessed 25 September, 2010. Return to text.
- Aldridge, S., The Thread of Life, The story of genes and genetic engineering, Cambridge University Press, Cambridge, UK, pp. 47–53, 1996. Return to text.
- Alberts, B., Bray, D., Johnson, A. et al., Essential Cell Biology, An Introduction to the Molecular Biology of the Cell, Garland Publishing Inc., New York, USA, pp. 259–262, 1998. Return to text.


Readers’ comments
Comments are automatically closed 14 days after publication.