Journal of Creation 22(1):28–33, April 2008
Browse our latest digital issue Subscribe
Clarity and confusion
A review of The Edge of Evolution: The Search for the Limits of Darwinism
by Michael J. Behe
Free Press, New York, NY, 2007

This new book by Michael Behe, a follow-up to Darwin’s Black Box (DBB), has created somewhat of a storm amongst the faithful defenders of Darwin such as Richard Dawkins, Jerry Coyne and Kenneth Miller. They came out with all guns blazing to try to destroy the credibility of this book, presumably hoping that their dismissive vitriol would cause potential readers to skip reading it. Dawkins claimed that Behe had completely departed from the message of DBB, but Behe rightly says (p. 7), ‘my conclusions are ultimately the same’.
As far as the science goes, Behe’s book is well worth reading; Behe argues very cogently that random mutations and natural selection are capable of very little (hence ‘the edge of evolution’) and cannot explain the major features of living organisms. Natural processes can explain variety at the level of species and perhaps genus and family and maybe order, but major class features and above are beyond the reach of natural processes. These features demand intelligent design. But that’s where Behe’s reasoning falls apart: he becomes quite incoherent as to what he means by intelligent design. But more on that later.
Clarity
Behe’s major argument as to where the edge of evolution lies (what random mutations can and cannot achieve), revolves mainly around the malaria parasite. He analyses the interaction between humans and Plasmodium falciparum. Plasmodium has mutated to overcome various antibiotics, such as chloroquine, and humans have mutated to generate some measure of resistance to malaria (e.g., sickle cell, thalassemia).
Behe shows that all the cases of adaptation, in both Plasmodium and humans, are due to breaking things, not creating new complex features. For example, chloroquine resistance in Plasmodium is due to a fault in a transport protein that moves the poison into the organism’s vacuole. Behe likens the struggle to trench warfare, where the defending forces will destroy their own bridge, or blow up a road, to impede the enemy’s advance. It is not really an arms race, because in an arms race the opposing forces invent new weapons, but the natural processes (‘evolution’) operating in Plasmodium and humans have not invented new weapons.
Chloroquine resistance—a relatively rare occurrence
Plasmodium, as a microbe, achieves huge populations and chloroquine has been around many years. This means that the parasite has had plenty of opportunity to undergo significant evolution, as the larger the total number of organisms, the more mutations that can be experimented with by natural selection.
However, resistance to chloroquine has only arisen relatively infrequently; it lasted a decade before resistance appeared. This contrasts with resistance to other anti-malarials, which has shown up within weeks of their first use. Behe points out that resistance to chloroquine involves 4–8 amino acids in a membrane transport protein (a ‘pump’). It seems to have arisen on four separate occasions. Two particular amino acid changes seem to be common (residues 76 and 220), so two mutations seem to be needed in the one gene. One mutation apparently does not generate any resistance to chloroquine, or must be compensated by a second mutation to overcome deleterious effects, whereas overcoming other anti-malarials has only needed one mutation for each one. This seems like a reasonable explanation for the relative resilience of chloroquine over time compared to other anti-malarials.
From this observation, Behe calculates, using the estimates of Plasmodium population numbers and generations provided by evolutionists, the probability of resistance requiring two mutations to occur where one is not helpful. One in 1020 parasites will have chloroquine resistance (p. 59). A very sick person will have 1012 parasites and if a billion people per year were infected, this gives 1021 parasites, which means we would expect at least one person per year to be infected by a parasite that has acquired resistance to chloroquine. These calculations are consistent with the observed resilience of chloroquine.
Slow-coach humans
Compared to Plasmodium, humans are boring when it comes to the evolutionary possibilities, because of our relatively small population and long generation times. Behe calculates, assuming the evolutionary timescale (which he does not question), the maximum total number of humans since the supposed split from chimps as 1012 individuals. Therefore it would take a billion years to have a chance of getting a double mutation like that needed for chloroquine resistance in Plasmodium. In other words, anything more difficult than this would never happen in a human-like organism.
Sickle cell trait, which requires a specific nucleotide change (mutation), has arisen de novo only a few times in human history. However, thalassemia, which merely requires the breaking of a hemoglobin gene, for which there are many ways to achieve this, has arisen hundreds of times. Again these frequencies are consistent with the probabilities of mutations in a human-like population. ‘Evolution’ (mutations and natural selection) is quite able to do this sort of thing.
If one in 1020 malaria parasites will have a double mutation, the chances of getting two such double mutations would be one in 1040. But this exceeds the total number of cells that have existed on earth in the billions of years that life is supposed to have existed. In other words, evolution could never achieve this. This is basically ‘the edge of evolution’ the limit to what mutations and natural selection can achieve.
Powerhouse yeast
Behe also looks at the history of yeast, again assuming the usual evolutionary scenario that yeast underwent a genome duplication hundreds of millions of years ago. He observes that no novel complexities have been added since. With the population numbers and short generation times, mutations have had plenty of opportunity to be creative, but … nothing.
He also looks at pyrimethamine resistance in Plasmodium, DDT resistance in mosquitoes and warfarin resistance in rats. In every case things are broken by mutations to create resistance.
Behe spends some time looking at anti-freeze in a fish (pp. 77–81). He acknowledges that the ‘evolutionary’ (mutations + selection) scenario painted is feasible. However, he points out that the protein fragments that comprise the antifreeze are quite non-specific, with no secondary structure and have no interaction with other proteins. All they have to do is interact with water molecules to inhibit crystallization.
They are of different lengths, from different genes, and can be regarded as the accumulation of ‘genetic debris’ that happens to be adaptive. He likens it to various pieces of wood, bark and leaves creating a dam in a creek: it can be done incrementally, and almost anything will do to add to the dam. This is the sort of thing that random changes can achieve. As Behe says, ‘Rare examples such as the Antarctic fish set Darwinian pulses racing. But to more sceptical observers, they underscore the limits of random mutation rather than its potential.’ Behe quotes one group of anti-freeze researchers: ‘A number of dissimilar proteins have adapted to the task of binding ice. This is atypical of protein evolution’ (p. 82).
Revisiting irreducible complexity
Behe revisits the cilium (pp. 84–96), discussed in Darwin’s Black Box as an example of irreducible complexity—a biological feature that could not be built by ‘numerous successive slight modifications’ (Darwin) because it has some 200 different protein components. Not only the components have to be explained, many of which are peculiar to the cilium, but also their precision assembly. Exciting discoveries since DBB make the problem even worse for Darwinian naturalism, particularly the realisation that intra-flagellar transport (IFT) occurs (transport of proteins within the flagellum itself) and is necessary for cilium functionality. IFT entails the active movement of protein components, by linear motors called kinesins ‘walking’ along microtubules, for repair of the cilium tip, so that each cilium is continuously rebuilt. Mutations that break IFT result in non-viability because cilia are necessary for such things as embryo development and eye and kidney function. Behe says, ‘IFT exponentially increases the difficulty of explaining the irreducibly complex cilium’ (p. 94).

He also revisits the bacterial flagellum, which is comprised of about three dozen proteins (figure 1). Much more has also been discovered about this, and it is also much more complex than previously envisaged (pp. 97ff). Behe gives a sketch of the marvellous control systems that are involved in achieving a ‘just-in-time’ organisation of construction of the various parts. It is precision engineering that no engineer would attribute to random changes (i.e. evolution).
There is just no way that a series of successive slight modifications could create such intricate machinery because dozens of components and steps are necessary before any functionality would be possible; there is no series of intermediate states that work to some extent and can therefore be favoured by natural selection. Such ‘coherent’ (a word used often by Behe) features of living things are well ‘past the edge of evolution’, says Behe (p. 102).
In contrast, where successive mutations are all beneficial, evolution can result in beneficial change. For example, the addition of the C-harlem mutation (β-hemoglobin residue 73) to the sickle mutation (residue 6) restores the decreased viability due to the sickle mutation. Based on a mutation rate of 30 per three billion nucleotides of the human genome per baby, with one of those being in protein-coding genes, Behe calculates that 1 in 100 million (1 in 108) babies will be born with a specific mutation, so this is achievable sequentially, but if both are needed together to be useful, the odds become 1 in 10 million billion (1016), which would never happen, even if a human population of one million had been around for a billion of years. This is beyond the edge of evolution.1
This is the problem of coherence: evolution can have no goal, but can only select what is helpful for survival now.
Upping the ante
Life is much more than a couple of mutations in a given gene (which of course has to pre-exist for the mutations to occur in it). Behe points out that life depends on many protein complexes where multiple proteins bind together in specific ways. Hemoglobin needs four proteins (two α and two β chains) to assemble correctly. The α-and β-globin chains are encoded on genes on different chromosomes, so they are expressed independently. This expression must be controlled precisely, otherwise various types of anemia called thalassemia result. Also, there is an essential chaperone protein called AHSP (alpha hemoglobin stabilizing protein) which, as the name implies, stabilizes the α-chain and also brings it to the β-chain. Otherwise the α-chain would precipitate and damage the red blood cells.2
But many functional proteins need six or more chains to bind in a specific manner. Behe concludes, justifiably, I believe, that,
‘Generating a single new cellular protein-protein binding site is of the same order of difficulty or worse than the development of chloroquine resistance to the malarial parasite. … the great majority of proteins in the cell work in complexes of six or more. Far beyond that edge [of evolution]’ (p. 135).
The fact that no novel protein-protein interactions have developed in the war between malaria and humans—in humans or the parasite—reinforces the point.
What about something like HIV that ‘mutates at the evolutionary speed limit’, 10,000 times the rate of Plasmodium? Although it has produced some 1020 copies in the past decades, its basic genetics have changed very little. ‘Each and every possible single point mutation occurs between 10,000 and 100,000 times per day in a HIV-infected individual.’ But this has produced ‘no new gizmos or basic machinery’ (p. 138). ‘No gene duplication has occurred leading to new function. None of the fancy tricks that routinely figure in Darwinian speculations has apparently been much use to HIV’ (p. 139).
The biochemical changes in both Plasmodium and HIV to overcome drugs have all been trivial, such as a point mutation slightly changing the shape of an enzyme.
Behe summarizes the statistical problems for the evolutionary scenario in an important Table 7.1 (p. 143). A typical cell might have some 10,000 protein-binding sites, but in all the cases surveyed, perhaps one protein-protein binding site has arisen by mutation: the sickle cell condition in humans, which is quite non-specific and actually destroys the normal structure of the hemoglobin tetrad and causes disease not evolutionary advancement. So Behe is being generous in granting one example. Considering the number of organisms that have ever lived, it is clear that evolution could have created only two at most of the ~10,000 binding sites. And definitely no complexes of three or more proteins (of which there are many).
The numbers of Plasmodium and HIV in the last 50 years would probably greatly exceed the total number of mammals since their supposed evolutionary origin (several hundred million years ago), yet little has been achieved by evolution. This suggests that mammals could have invented little in their time frame. Behe: ‘Our experience with HIV gives good reason to think that Darwinism doesn’t do much—even with billions of years and all the cells in that world at its disposal’ (p. 155).
Ruses answered
Behe deals with attempts to give naturalism a leg up, such as Stuart Kauffman’s ‘complexity theory’ where things are supposed to have an innate tendency to self-organize (p. 159). But the very same evidence adduced to show that random mutations cannot create the grand organizational complexity of living organisms also shows that there is no tendency to self-organize. There is no innate ability of living things to create new specified complexity (such as multiple protein binding sites).
The same goes for James Shapiro’s idea that cells have the ability to create new functions due to a built-in toolbox that allows for self genetic engineering. Of course this would mean that the basic, original living thing that had the toolbox would be even more fantastically complex than it is known to be. But the evidence that Behe presents argues against any such ability also.
The discovery that certain common genes (e.g. Hox genes) control the basic body plans of a wide range of creatures excited evolutionists with imagined possibilities for evolution. For example, very similar genes control where eyes form in both insects and vertebrates, although the eye designs are quite different. Such discoveries launched the ‘Evo Devo’ movement. Evolutionists thought that a little tinkering (mutations) with the Hox genes could generate new body plans and even explain the origin of major categories of life, for example. However, the initial hype has not been fulfilled. Mutations in these genes do not generate anything fundamentally new; they just rearrange what already exists (putting eyes or antennae where they would not normally be, for example). So this has been a dead end in terms of evolution. And as Behe points out, the discovery of control systems makes the problem for evolution even worse—not only does evolution have to explain the origin of the protein-coding genes, but their control systems as well.
In an important aside, Behe points out that Darwinism did not predict molecular homology (quoting François Jacob, Ernst Mayr and Sean Carroll), nor anticipate the discoveries relating to common developmental controls (quoting Sean Carroll, Kirschner and Gerhart, and Walter Gehring). Behe says, ‘Time and again, by intentionally reasoning about animal life on Darwinian principles, the best minds in science have been misled.’ Evolution is bad for science.
The problem of the evolution of control systems is underlined with the discovery of gene regulatory networks (GRNs) that specify the sequence of steps needed to build an animal’s body components. These are logic maps like those used in designing computers (p. 196) that involved dozens of protein-switches and proteins. Failure of one causes failure of the whole—they are irreducibly complex also. Modules of these GRNs are called ‘kernels’. One kernel, for example, specifies the steps in constructing one of the layers in a sea urchin.3 Behe points out that different phyla differ in their body plans, so must have different kernels, which could not evolve. So the origin of phyla is beyond the edge of evolution.
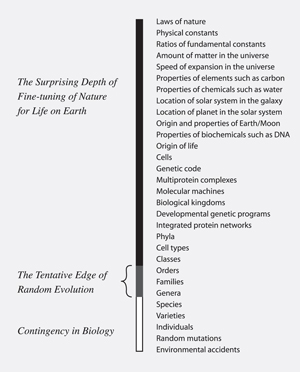
He then goes on to point out that specific cells such as the B cells of our immune system are controlled by a GRN at least as complex as the one elucidated in sea urchins. Since different vertebrate classes differ in the numbers of different cell types that are produced—e.g. amphibians about 150, birds 200 and mammals 250 cell types, this strongly suggests that the origin of these classes is beyond the edge of evolution.
Shades of baraminology
So Michael Behe comes to the grand conclusion to his survey: ‘Somewhere between the level of vertebrate species and class lies the organismal edge of Darwinian evolution’ (p. 201). A diagram illustrates this (p. 218), which he reproduces on the page facing the title page of the book (figure 2).
Interestingly, the creationist study of baraminology (defining the limits of the original created kinds, or baramins, of Genesis 1) has arrived at conclusions consistent with Behe’s proposition, using a different approach based on hybridization criteria, where possible, combined with morphology, etc.4 In fact, in 1976 creationist biologist Frank Marsh proposed that the created kinds (baramins) were often at the level of genus or family, although sometimes at the level of order.5
Confusion
What does Behe propose instead of random mutations to explain the origin and diversity of life? What does he mean by intelligent design? That’s where things get rather muddy.
Behe looks at the fine-tuning of the universal constants and laws of physics that govern the properties of our universe. He rightly dismisses the ‘multiverse’ idea as an explanation for the ‘just-right’ conditions for the existence of life on our planet in our universe. It does not rescue Darwinism as an inadequate explanation for biological complexity (p. 222).
He accepts the collision hypothesis for the origin of the moon (pp. 212–3) and since it is necessary for life on earth (tides, etc.) suggests that the collision was somehow ‘purposely arranged’ (p. 215). He overlooks the many problems with this idea.6
He also states that the origin of life was ‘deliberately, purposely arranged’ (p. 216) and that intelligence is needed to explain the origin of biological features involving two or more protein binding sites.
Interestingly, Behe initially refers to the designer impersonally (‘it’, ‘which’) but later slides into references using the personal pronoun (‘who’).
Behe criticizes theistic evolutionists who think that this is compatible with Darwinism, because he says if an external agent set up the universe with properties that would ensure a particular outcome, then it was not due to random variation and it is therefore not Darwinism. Also, the physical laws describe how matter behaves in general terms and do not of themselves contain the specificity to create life, for example. In other words there is no ordained outcome from just the operation of the physical laws.
And then it gets really confusing. Behe says that science has revealed a fine tuning ‘well beyond laws, past details, into the very fabric of life’ (p. 230). ‘But the assumption that design unavoidably requires “interference” rests mostly on a lack of imagination. There’s no reason that the extended fine-tuning view I am presenting here necessarily requires active meddling with nature any more than the fine-tuning of theistic evolution does. One can think the universe is finely tuned to any degree and still conceive that “the universe originated by a single creative act” and underwent “its natural development by laws implanted in it.” One simply has to envision that the agent who caused the universe was able to specify from the start not only laws but much more.’
But just what mysterious thing did the designer specify ‘from the start’? Behe does not say. He says, ‘Those who worry about “interference” should relax. The purposeful design of life to any degree is easily compatible with the idea that, after its initiation, the universe unfolded exclusively by the intended playing out of natural laws’ (p. 232). This sounds like theistic evolution or Kauffman’s innate self-organization, both of which Behe himself effectively countered. The data that he so elegantly presented show that there is no tendency of living things to create new specified complexity, by random or non-random processes—by any natural process.
Behe also seems confused about common descent, which he repeatedly asserts that he accepts. He accepts the evolutionists’ argument that shared supposed DNA errors demonstrate common descent (that humans and chimps had a common ancestor, for example). But the argument depends on the assumption that so-called ‘pseudogenes’ are functionless. Behe himself inadvertently provides the evidence against the idea, in discussing the prevalence of mutations (p. 68). If we assume the evolutionary scenario of millions years since our split from a common ancestor with chimps, if the ‘pseudogene’ were functionless, unconstrained by natural selection, then it should have mutated almost beyond recognition. But it hasn’t—the close similarity is claimed as evidence of common ancestry! So, using the evolutionists’ own assumptions, it is not ‘useless’ and the evolutionary argument disintegrates and the similarity becomes evidence for common design, not common ancestry.7
Dr Behe even weighs in on the issue of natural evil and its implications for his intelligent designer—was ‘the designer of life a dope, a demon, or a deity’? Biblical creationists have consistently pointed to this gaping hole in the vaguely theistic worldview of the ID movement that excludes revelation (the Bible) from consideration, and thus the Fall.8 Behe doesn’t really try to answer the question, merely saying that science can’t answer such questions (p. 239).
The critics
The heavyweights of the Darwinian faithful have weighed in on criticising Behe’s book. Most of their vitriol amounts to little more than abusive ad hominem attacks, but to the occasional scientific criticism, Behe has responded quite ably.9 It does not appear that Richard Dawkins even read the book before going into print—he cited the variety in domestic breeds of dogs as proving the sufficiency of mutations to generate the diversity of life (Behe clearly regards such variation as within the ability of random mutations and selection, as do biblical creationists).10
Overall, this book is a valuable contribution to the debate over the sufficiency of random mutations to account for the origin and diversity of life on earth, but the author seems confused as to what he means by intelligent design.
References
- The mutation rate is actually much higher, 100–300 per person, but that is insufficient to rescue the evolutionary problem Behe outlines. Furthermore, this higher rate means that evolution has another serious problem: that natural selection is powerless to remove deleterious mutations from the human population, so that we are in mutational meltdown. This puts severe limits on the age of the human population. See Sanford, J.C., Genetic entropy and the mystery of the genome, Elim Publishers, New York, 2005. Return to text.
- Kihm, A. et al., An abundant erythroid protein that stabilizes free-haemoglobin, Nature 417(6890):758–763, 13 June 2002; comment by L. Luzzatto and R. Notaro, Haemoglobin’s Chaperone, same issue, pp. 703–705. Return to text.
- See The Davidson Lab: Endomesoderm Gene Network; <sugp.caltech.edu/endomes>, 13 Dec. 2007. Return to text.
- Wood, T., A baraminology tutorial with examples from the grasses (Poaceae), TJ (now Journal of Creation) 16(1):15–25, 2002. Return to text.
- Marsh, F.L., Variation and Fixity in Nature, Pacific Press, CA, 1976. Return to text.
- See Sarfati, J., The moon: the light that rules the night, Creation 20(4):36–39, 1998; <creation.com/moon>; Henry, J., The moon’s recession and age, J. Creation 20(2):65–70, 2006. Return to text.
- See also, Woodmorappe, J., Potentially decisive evidence against pseudogene ‘shared mistakes’, TJ (now Journal of Creation) 18(3): 63–69, 2004. Return to text.
- Wieland, C., CMI’s views on the Intelligent Design Movement, 2002; <creation.com/idm>. Return to text.
- See Behe’s blog on Amazon.com, accompanying information about the book. Return to text.
- Sarfati, J., Misotheist’s misology: Dawkins attacks Behe but digs himself into logical potholes, 2007; <creation.com/dawkbehe>. Return to text.


Readers’ comments
Comments are automatically closed 14 days after publication.