The non-evolution of the horse
Special creation or evolved rock badger?

Probably no animal has been as important to human history as the horse. Before the steam and petrol engines were invented, they were the fastest form of transport on land. Their use by messengers and soldiers has turned the tide of many a battle. And they have many other uses. Several cultures drink mare’s milk; and horse hair is used for violin bows, mattresses and lining for clothes.
Even their immune systems produce life-saving tetanus anti-toxin, while their manure is commonly used for fertilizer and was sometimes even used for fuel. Horsehide is made into fine cordovan leather, and glue is often made by boiling horse bones and cartilage.1,2 Finally, many people ride horses for enjoyment.
The infamous ‘horse evolution’ series
For the last century or so, this fine animal has been put to a more unfortunate use. Its alleged ancestry has been used as one of the key ‘proofs’ of evolution. It started in 1879 with the American paleontologist O.C. Marsh and the famous evolutionist T.H. Huxley, known as ‘Darwin’s bulldog.’ Since then, many museums and popular books have presented a neat series starting from the dog-sized, four-toed ‘dawn horse’ or ‘Eohippus,’ which supposedly lived 50 million years ago. The next creature is usually a larger creature like Mesohippus, which had three toes. The next one was larger still, for example Merychippus, which had two of the toes smaller than the third. Finally, there is the large modern horse, Equus, with only one toe, while all that is left of the other two are ‘vestigial’ splint bones.3 Some of the diagrams also show trends in tooth changes, with increasing hypsodonty (high-crowned teeth). This is supposed to demonstrate a change from browsing on bushes to grazing on grass.
How clear-cut is it, really?
As the biologist Heribert-Nilsson said, ‘The family tree of the horse is beautiful and continuous only in the textbooks,’4 and the famous paleontologist Niles Eldredge called the textbook picture ‘lamentable’5 and ‘a classical case of paleontologic museology.’6
As shown in a detailed thesis by Walter Barnhart,7 the horse ‘series’ is an interpretation of the data. He documents how different pictures of horse evolution were drawn by different evolutionists from the same data, as the concept of evolution itself ‘evolved.’
This especially applies to reconstructing the animals from fossil skeletons, which are usually very incomplete. The evolutionist Gerald Kerkut wrote:
‘It takes a great deal of reading to find out for any particular genus just how complete the various parts of the body are and how much in the illustrated figures is due to clever reconstruction. The early papers were always careful to indicate by dotted lines or lack of shading the precise limits of the reconstructions, but later authors are not so careful.’8
Informed evolutionists now realize that the picture, even in their own framework, is not a straight line at all. While they still believe in horse evolution, the modern view of the horse fossil record is much more jumpy and ‘bushy.’9
What is the ‘dawn horse’?
This creature was discovered in 1841 by Richard Owen, one of the leading paleontologists of the day, the inventor of the word ‘dinosaur,’ and a staunch opponent of Darwin. Owen saw no connection with the horse, but thought it was very like a modern-day hyrax—that is, a rock badger or coney. So he named it Hyracotherium. Other fossils of the same type of creature were later named ‘Eohippus’ or ‘dawn horse’ by more evolutionarily-minded paleontologists. But the name given by the discoverer takes priority. Thus ‘it is not clear that Hyracotherium was the ancestral horse’, according to Kerkut.10
The fossils
The fossils do not carry signs saying how old they are. Their age is generally assigned to them, depending on their relative depth of burial. Those in the deepest rock layers have the greatest ages assigned to them. Based on the biblical framework, we should expect many, but not all, fossils to have been buried during the Flood, so the oldest would really be only about 4,500 years old. Fossils higher up may have been buried by local catastrophes since the Flood.
It’s likely that many of the horse fossils were post-Flood. However, even if we were to grant the evolutionary/long age dating, they don’t show the clear progression presented by the textbooks. For example, in north-eastern Oregon, the three-toed Neohipparion and one-toed Pliohippus were found in the same layer. This indicates that they were living at the same time, and thus provides no evidence that one evolved from the other.11,12
Lots of different horses

Living horses come in a wide range of sizes. Their heights are usually measured in hands. One hand = 10 centimeters (4 inches). The largest is the English Shire horse, which can measure as much as 20 hands.1 Ponies are horses under 14.2 hands,1 and the Fallabella is just over four hands when fully grown.
Horses vary in other ways too. Modern horses can have 17, 18 or 19 pairs of ribs. Also, three-toed horses are known today. O.C. Marsh himself noted that some horses in the American southwest had three toes of almost equal size, ‘thus corresponding to the feet of the extinct Protohippus.’13
An important part of the biblical creation model is that different kinds of creatures were created with lots of genetic information. Natural selection can sort out this pre-existing genetic information, by eliminating creatures not suited to a particular environment. Thus many different varieties can be produced in different environments. Note that this sorting process involves a loss of information, so is irrelevant to particles-to-people evolution, which requires non-intelligent processes to add new information.14,15
Also, much of this (created) genetic information may have been latent (hidden, i.e. the features coded for are not expressed in the offspring) in the original created kinds. They also had other controlling or regulatory genes that switch other genes ‘on’ or ‘off.’ That is, they control whether or not the information in a gene will be decoded, so the trait will be expressed in the creature. This would enable very rapid and ‘jumpy’ changes, which are still changes involving already created information, not generation of new information.
Applying these principles to the horse, the genetic information coding for extra toes is present, but is switched off in most modern horses. Sometimes a horse is born today where the genes are switched on, and certainly many fossil horses also had the genes switched on. This would explain why there are no transitional forms showing gradually smaller toe size.
It’s possible that body size and tooth shape were also controlled by regulatory genes.16 This is supported by an experiment by Paul Sharpe and his colleagues on mouse embryos. They found that a single protein, called BMP-4, inhibits the gene that causes molars (back grinding teeth) to form, so incisors (cutting teeth) can grow instead. Without this protein, no incisors grew.17
These mechanisms would explain the alleged horse evolutionary series as variation within the equine (horse) kind. The amount of variety within living horses, undoubtedly one kind, supports this.
Tooth shape
Certainly tooth shape can vary widely within a kind, meaning that it’s unwise to assume that different fossil teeth show evolution.18 It is also unwise to be dogmatic about diets based on tooth shape. We showed this with bats,19 and recent evidence has overturned previous thought about ancient horse diets based on tooth shape. The evolutionary paleontologist Bruce MacFadden analyzed teeth from six horse ‘species’ (more likely, varieties within a kind), ‘dated’ at five million years ago.20
Previous evolutionary theories would have asserted that because they all had high-crowned teeth, they must have been grazers. But the amounts of stable carbon isotopes 12C and 13C impregnated into the teeth indicated that the horses were browsers, not grazers.
The researchers also claimed that once hypsodonty evolved, it was impossible to return to having short-crowned teeth again. In a creationist model, this suggests that hypsodonty is a highly specialized condition, which has lost genetic information for any other sort of teeth.
Again, this information loss is the opposite of molecules-to-man evolution, much like the long-furred bears in the diagram of Ref. 15.
Splint bones: useless leftovers or good design?

Many evolutionists claim that the horse’s splint bones in their legs (see diagram right) are vestigial, that is, useless leftovers from its alleged evolutionary past. But the evolutionary zoologist Scadding pointed out, ‘vestigial organs provide no evidence for evolutionary theory.’21
He pointed out that the argument is unscientific, because it is impossible in principle to prove that an organ has no function; rather, it could have a function we don’t know about.22 Scadding also reminds us that ‘as our knowledge has increased the list of vestigial structures has decreased,’ and pointed out that the 19th century claim of hundreds has been shrunk to a handful of doubtful cases.23 Also, at best, vestigial organs could only prove devolution (loss of information), not evolution.
In particular, the horse’s splint bones serve several important functions. They strengthen the leg and foot bones, very important because of the enormous stress that galloping puts on the legs. They also provide attachment points for important muscles. And they form a protective groove that houses the suspensory ligament, a vital elastic brace that supports the horse’s weight as it walks.24
The horse shows that similarities are due to creation!
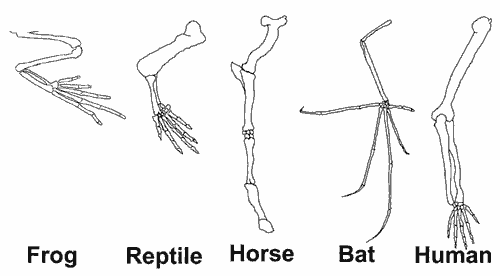
Evolutionists claim that similarities in the limbs of frogs, reptiles and mammals show that they all evolved from a common ancestor. Amphibians (e.g. frogs) supposedly gave rise to reptiles, which gave rise to mammals, including bats and humans, hence the similarities in their limb structures. However, the horse’s leg doesn’t fit very well into this ‘explanation.’
The horse is much more similar to humans in other respects than a frog, but the frog’s limb is much more like ours. The evolutionist tells a story here to ‘explain’ this discrepancy: the horse is different because its legs became adapted to a different way of walking. This is ‘just-so’ story-telling, not science.
Perhaps the horse is part of the pattern God created to tell us there is one Creator (the similarities in living things) but that things did not make themselves (there are oddities which don’t fit any ‘everything made itself’ story).
Furthermore, the frog embryo develops its legs differently from us—amphibian digits develop by bud growth outwards, while amniote (reptile, bird and mammal) digits are formed as parts of a bony plate are dissolved in between. Yet they arrive at a similar pattern, again indicating the hand of a master designer rather than chance.25 There really is no excuse (Romans 1:20).
Summary
- The textbooks create this ‘evolutionary series’ from a probable non-horse (Hyracotherium) and varieties of true horses.
- Far from being an example of evolution, it is an example of the wide variation within a created kind.
- Particles-to-people evolution requires new information to be generated, while the horse varieties, especially in number of toes, result from pre-existing information being switched on or off, as well as natural selection removing information.
- Theories of adaptation to different diets based on tooth shape have been undermined by recent isotopic analysis.
- The ‘splint bones,’ far from being useless vestiges of evolution, play an important role in the horse’s leg.
References and notes
- ‘Horse and horsemanship,’ Encyclopædia Britannica, 20:646–655, 15th Ed. 1992. Return to text.
- Of course, some of these uses could only have taken place after Adam’s sin brought death into the creation (Romans 5:12, 8:20–22, 1 Corinthians 15:21–22); see The Fall: a cosmic catastrophe. Return to text.
- ‘Evolution, the Theory of,’ Encyclopædia Britannica, 18:855–883, 15th Ed. 1992—see p. 861. Return to text.
- Heribert-Nilsson, Synthetische Artbildung, Gleerup, Sweden, Lund University, 1954; cited in Luther Sunderland, Darwin’s Enigma: Fossils and Other Problems, 4th Ed., Master Books, Santee, CA, p. 81, 1988. Return to text.
- Eldredge, N., quoted by Sunderland, ref. 4, p. 78. Return to text.
- Eldredge, N., Life Pulse: Episodes from the story of the fossil record, Penguin, London, p. 222, 1989. Return to text.
- Barnhart, W., ‘A critical evaluation of the phylogeny of the horse,’ M.Sc. Thesis, Institute for Creation Research, Santee, CA, 1987. Return to text.
- Kerkut, G.A., Implications of Evolution, Pergamon Press, London, New York, p. 146, 1960. Return to text.
- The palaeontologist David Raup wrote: ‘The record of evolution is still surprisingly jerky and, ironically, we have even fewer examples of evolutionary transition than we had in Darwin’s time. By this I mean that the classic cases of darwinian change in the fossil record, such as the evolution of the horse in North America, have had to be modified or discarded as a result of more detailed information. What appeared to be a nice simple progression when relatively few data were available now appears to be much more complex and less gradualistic. So Darwin’s problem has not been alleviated.’ D.M. Raup, ‘Conflicts between Darwin and paleontology,’ Field Museum of Natural History Bulletin 50:22, 1979. Return to text.
- Ref. 8, p. 149. Return to text.
- Gish, D, Evolution: The fossils STILL say NO!, Institute for Creation Research, El Cahon, CA, USA, pp. 187–197, 1995. Return to text.
- However, some creationists believe that there really is a trend in the fossil record. They believe this reflects adaptation within the horse kind to a change from woodland to grassland, caused by cooling and drying of the post-Flood Earth. They point out that these climatic changes are difficult to explain under an evolution/billions of years scenario. See P. Garner, ‘It’s a horse, of course! A creationist view of phylogenetic change within the equid family,’ Origins (Journal of the Biblical Creation Society) 25:13–23, 1998. This was written before Ref. 20. Return to text.
- Marsh, O.C., ‘Recent polydactyle horses,’ American Journal of Science 43:339–354, 1892. Return to text.
- See Carl Wieland, Beetle bloopers, Creation 19(3):30, 1997. Return to text.
- Weston, P., and Wieland, C., Bears across the world, Creation 20(4):28–31, 1998. Return to text.
- Brand, L., Faith, Reason and Earth History: A paradigm of earth and biological origins by intelligent design, Andrews University Press, Berrien Springs, MI, USA, p. 202, 1997. Return to text.
- Tucker, A.S., Matthews, K.L., Sharpe, P., ‘Transformation of tooth-type induced by inhibition of BMP signaling,’ Science 282(5391):1136–1138, November 6, 1998. BMP = Bone Morphogenetic Protein. Return to text.
- García-Pozuelo-Ramos, C., ‘Dental variability in the domestic dog (Canis familiaris): Implications for variability of primates,’ Creation Research Society Quarterly 35(2):66–75, 1998. Return to text.
- Weston, P., Bats: sophistication in miniature, Creation 21(1):28–31, 1998; but the online version lacks the picture of the bats’ skulls in the magazine. Return to text.
- MacFadden, B.J., et al., Ancient diets, ecology, and extinction of 5-million-year-old horses from Florida, Science 283(5403):824–827, 5 February 1999. See also comments on p. 757 and 773 of the same journal. Return to text.
- Scadding, S.R., ‘Do vestigial organs provide evidence for evolution?’ Evolutionary Theory 5:173–176, 1981. Return to text.
- The Shorter Oxford English Dictionary (1993) defines ‘vestigial’ as ‘degenerate or atrophied, having become functionless in the course of evolution.’ Some evolutionists now re-define ‘vestigial’ to mean simply ‘reduced or altered in function.’ Thus even valuable, functioning organs (consistent with design) might now be called ‘vestigial.’ Creationists should not let evolutionists change the rules when they lose. Return to text.
- R. Wiedersheim claimed that there were over 180 rudimentary organs in the human body, of which 86 were vestigial, in The Structure of Man: an Index to his Past History; translated by H. and M. Bernard, Macmillan, London, 1895. Return to text.
- Bergman J., and Howe, G., ‘Vestigial Organs’ Are Fully Functional, Creation Research Society Books, Kansas City, p. 77, 1990; Murris, H.R., ‘Vestigial organs: A creationist re-investigation,’ Origins (Journal of the Biblical Creation Society) 5(13):10–15, 1992; see also ‘Vestigial’ organs: What do they prove? Return to text.
- ReMine, W.J., The Biotic Message, St. Paul Science, St. Paul, MN, USA, 1993; see review. Return to text.





Readers’ comments
Comments are automatically closed 14 days after publication.