Journal of Creation 35(1):3–5, April 2021
Browse our latest digital issue Subscribe
Phoenicoid fungi: first responders at Mount St Helens

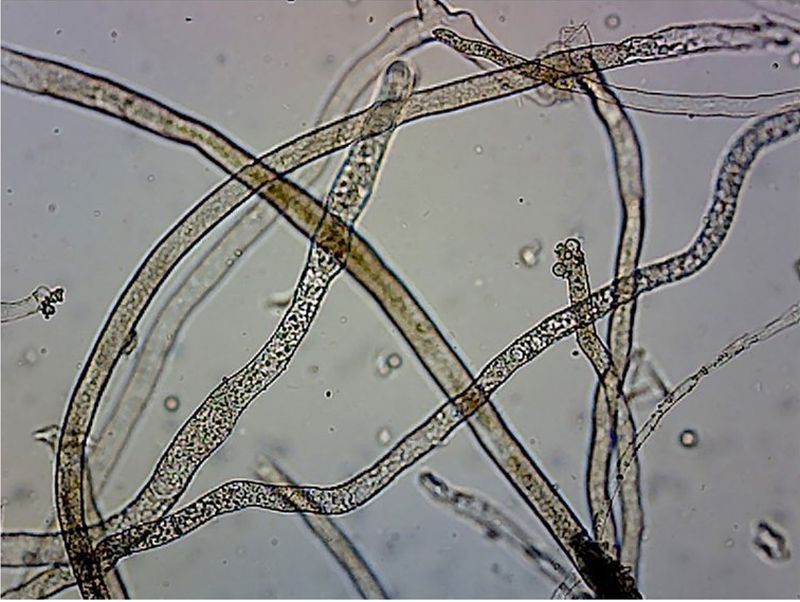
On 28 May 1980, just 10 days after the cataclysmic eruption of Mount St Helens in the state of Washington, USA, geomorphologist Fred Swanson was digging ‘little holes’ in the newly emplaced debris avalanche deposit.1,2 Located immediately north of the volcano, the deposit consisted of landslide material from the collapse of the summit and north slope of Mount St Helens, covered by pumice fragments from the subsequent nine-hour eruption. As he worked, Swanson noticed something that puzzled him. Extending from the wall of one of the holes and fluttering in the breezes were barely visible spiderweb-like filaments, to which tiny ash particles had adhered. Swanson did not understand what he was seeing, but later learned the filaments were hyphae3 (figure 1) of so-called ‘burn-site’, ‘fire’, or ‘pyrophilous’ fungi.4 These organisms are known to persist for decades and only produce fruiting bodies (‘mushrooms’) after the heat5 of a fire6—or in this case, a volcanic eruption. Swanson dubbed them ‘the first biological response’ to the eruption.7 And since fire was not the heat source, mycologists Steven E. Carpenter and James M. Trappe later coined a more general term ‘phoenicoid fungi’, after the mythical Phoenix bird (which arises from ashes),8,9 for “fungi that pioneer on heat-treated substrates”.10
Post-eruption fungi
During his initial mycological foray into the blast zone on 1 July 1980, Carpenter observed white patches of fungus growing in moist depressions on tephra11 deposits and in areas shaded by fallen trees.12,13 He identified this first observed fungal colonizer as Anthracobia melaloma (figure 2), a well-known phoenicoid fungus associated with forest fires. In addition, within some of the fungal colonies were algal filaments and the earliest stages of moss growth. Notably, these initial photosynthetic organisms were found only in association with A. melaloma colonies, not on barren pumice deposits. Over the ensuing months, numerous other species of phoenicoid fungi appeared, both as mycelial3 mats and as fruiting bodies. Within these fungal patches, algae, bryophytes (mosses), and vascular plants subsequently established, forming small oases of plant recovery within the pumice desert.
Remarkably, the pattern of fungal responses observed at Mount St Helens was similar to post-fire fungal behaviour in other parts of the world.6 For example, Anthrocobia species generally appear soon after a thermal stimulus, to be followed by multiple other ascomycete14 species and months later by basidiomycetes.15 Claridge et al comment:
“ … postfire fungal phenomenon [sic] occur similarly halfway around the world in forest types as dissimilar as Pacific Northwestern conifers and south-eastern Australian eucalypts”6
“The parallels between the fires in these far removed and markedly different forests were striking, as was the similar mycological aftermath of the Mount St Helens eruption.”6
Ecological roles

The early arrival of phoenicoid fungi played several pivotal roles in “laying the ecological foundation for a new ecosystem”7 following the 1980 eruption of Mount St Helens. Listed below are processes attributed to phoenicoid fungi based on observations at Mount St Helens and other sites. They:
1. Stabilized substrates
Immediately following their emplacement, loose deposits of tephra were highly erodible, lacking the stability needed for plant recovery. The rapid growth of fungal hyphae within these deposits acted like living, microscopic rebar, stabilizing substrates. Claridge et al. summarize:
“We hypothesise that fungi such as Anthracobia are pivotal species in early system recovery after disturbance, helping minimize the movement of soil in the absence of plant roots.”16
2. Increased aeration and water infiltration
In addition to stabilizing post-eruption substrates, fungal hyphae aggregated small particles of tephra, forming pore spaces, which facilitated the entry of air and water into developing soils, benefiting plants. Claridge et al. state concerning postfire fungi: “Mycelial networks bind soil particles into aggregates, thereby improving aeration and water infiltration.”6,17
3. Decomposed organic materials
Much organic material was incorporated into the lateral blast as it penetrated the forest north of Mount St Helens.18 Soil, leaves, coarse woody debris, and animal remains permeated the resulting nutrient-deficient volcanic deposits.19 Subsequent decomposition of these organics by phoenicoid fungi released organically bound nitrogen, phosphorus, and other nutrients, creating a substrate more suitable for plant colonization than loose tephra.
Claridge et al. illustrate the critical need for nutrient acquisition by fresh volcanic substrates with the following discovery, which occurred about a year after the eruption:
“Fluids from the decaying remains of a horse killed by the eruption had seeped down slope. The seepage zone was a lush garden of bryophytes and fungal fruiting bodies set amidst the gray, non-vegetated tephra otherwise blanketing that site.”18
4. Sequestered nutrients
Nutrients released into volcanic deposits by fungal decomposition of organic materials were then taken up and sequestered by additional fungi, algae, mosses, and vascular plants. Subsequent decomposition of these organisms released more nutrients, further enriching developing soils.
5. Produced organic platforms for photosynthesizers
Sites where hyphal mats and masses of fruiting bodies of phoenicoid fungi had stabilized and enriched pumice substrates became prime locations for early colonization by photosynthesizers, including algae, mosses, and vascular plants. Carpenter et al. state: “The phoenicoid fungi on tephra in the devastation zone clearly prepared microsites for the growth of photosynthesizers”20 and “were clearly crucial in releasing nutrients from organic matter in the tephra for uptake by algae and bryophytes.”21
6. Likely formed symbiotic relationships with plants
Most forest plants in the Pacific Northwest (and elsewhere) form symbiotic associations with fungi, including phoenicoid species. Such relationships may be mycorrhizal, endophytic, or parasitic (pathogenic). Claridge et al., referring to phoenicoid fungi, state: “still others appear to be mycorrhizal symbionts with or pathogens on tree roots.”6
Mycorrhizal fungi are soil organisms that form mutualistic symbioses with plant roots. The fungal symbionts benefit from this association by receiving photosynthesized carbon compounds from the plants. In turn, fungal symbionts act as extenders of plant roots bringing soil nutrients (including phosphorous, magnesium, iron) and water to their host plants. The importance of mycorrhizae cannot be overemphasized. Allen states: “Mycorrhizae can be formed between nearly all phyla of fungi and almost all land plants, with only a few exceptions.”22 At Mount St Helens, all forest tree species are dependent on fungal associations for normal growth and development.
Endophytic fungi, some of which are phoenicoids, are plant symbionts that reside within plant tissue and even within plant cells. The association is often mutualistic in that the fungal symbionts receive carbon compounds from their host plants, which in turn are defended against arthropod herbivores by chemicals produced by the fungi.23 For example, the dominant conifer tree of many forests in north-western North America is the Douglas fir (Pseudotsuga menziesii). Its needles are inhabited by several species of fungi capable of mounting chemical attacks on species of defoliating insects.24
Some fungi associated with plant roots, as well as certain endophytic fungi, are parasites or pathogens of plants. Although detrimental to their host plants, they help control the growth and spread of plant populations, preventing species from becoming invasive.
7. Provided a food source for animals
Although not well-documented in the literature, phoenicoid fungi likely served as an important food source for colonizing fungivorous insects and small mammals.
Implications
In ecology, a ‘disturbance’ is any process that disrupts an ecosystem, such as fire, hurricane, landslide, or volcanic eruption. Immediately following such a disturbance, a suite of processes called ‘succession’ (or in lay terms, ‘recovery’) is initiated. It appears that ecosystems are well-designed by their Creator to respond effectively to disturbances, even catastrophic ones. Such a remarkable ability to bounce back is a clear indication of design in ecosystems, just as there is design in cells, organs, and organisms. The rapid response of phoenicoid fungi to fire or volcanic eruption provides good evidence of the Creator’s forethought.
Phoenicoid fungi also provide insight into ecological responses that occurred following Noah’s Flood. Certainly, propagules of phoenicoid fungi survived the Flood in the atmosphere, the floodwaters, on floating vegetation mats, and in sediments. Post-Flood volcanic eruptions, hydrothermal processes, and fires would have provided heat sources. Similarities between heat-induced fungal responses in diverse parts of the world today suggest these responses are normative following fire or volcanic events. It is reasonable, therefore, to hypothesize that activities involving phoenicoid fungi, similar to those documented at Mount St Helens, occurred globally on heat-affected substrates in the aftermath of Noah’s Flood.
References and notes
- Luoma, J.R., The Hidden Forest: The biography of an ecosystem, Oregon State University Press, Corvallis, OR, pp. 13–15, 1999. Return to text.
- Wagner, E., After the Blast: The ecological recovery of Mount St. Helens, University of Washington Press, Seattle, WA, p. 23, 2020. Return to text.
- ‘Hyphae’ are fungal filaments, composed of cell material, that make up the body of a fungus; a network of hyphae is termed a ‘mycelium’. Return to text.
- Propagules (spores and fungal fragments) of this fungus were likely transported to the debris avalanche deposit by turbulent winds associated with the lateral blast of the volcano, which ripped up and incorporated forest soil and vegetation. Propagules could also have survived in the debris avalanche itself, which was buried beneath the pumice deposits. Return to text.
- Besides heat, other factors may be involved in triggering germination of phoenicoid propagules, such as changes in pH and chemistry of the substrate, and loss of a nutritionally associated host plant. Return to text.
- Claridge, A.W., Trappe, J.M., and Hansen, K., Do fungi have a role as soil stabilizers and remediators after forest fire? Forest Ecology and Management 257:1064, 2009. Return to text.
- Luoma, ref. 1, p. 15. Return to text.
- Carpenter, S.E. and Trappe, J.M., Phoenicoid fungi: a proposed term for fungi that fruit after heat treatment of substrates, Mycotaxon 23:203–206, 1985. Return to text.
- Carpenter, S.E., Trappe, J.M., and Ammirati, Jr, J., Observations of fungal succession in the Mount St. Helens devastation zone, 1980–1983, Canadian J. Botany 65:717, 1987. Return to text.
- Carpenter and Trappe, ref. 8, p. 203. Return to text.
- Tephra is fragmented (pyroclastic) material erupted into the air from a volcano. Return to text.
- Carpenter et al., ref. 9, p. 718. Return to text.
- Geologists reported seeing white fungal patches (presumably A. melaloma) as early as June 12, 1980 (ref. 12). Return to text.
- Ascomycetes (also called ‘sac fungi’) are members of the fungal phylum (division) Ascomycota, which contains over 64,000 species. It is named after the ‘ascus’, a microscopic structure in which spores are formed. Examples include Anthracobia, morels, cup fungi, and some truffles. Return to text.
- Basidiomycetes (also called ‘club fungi’) are members of the fungal phylum (division) Basidiomycota, which contains over 31,000 species. It is named after the ‘basidium’, a microscopic structure in which spores are formed. Examples include typical mushrooms, puffballs, and bracket fungi. Return to text.
- Claridge et al., ref. 6, p. 1063. Return to text.
- In contrast, surface mycelia may also increase water repellency, which may promote erosion and decrease water infiltration (ref. 6). Return to text.
- Claridge et al., ref. 6, p. 1065. Return to text.
- Engle, M.S., Nitrogen and Microbial Colonization of Volcanic Debris on Mount St Helens, Masters Thesis, Washington State University, Pullman, WA, 1983. Return to text.
- Carpenter et al., ref. 9, p. 722. Return to text.
- Carpenter et al., ref. 9, p. 719. Return to text.
- Allen, F.A., O’Neill, M.R., Crisafulli, C.M., and MacMahon, J.A., Succession and Mycorrhizae on Mount St. Helens; in: Crisafulli, C.M. and Dale, V.H., (Eds.), Ecological Responses at Mount St. Helens: Revisited 35 years after the 1980 eruption, Springer, New York, pp. 199–215, 2018. Return to text.
- Hughes, K.W., Case, A., Matheny, P.B, Kivlin, S., Petersen, R.H., Miller, A.N., and Iturriaga, T., Secret lifestyles of pyrophilous fungi in the genus Sphaerosporella, American J. Botany 107(6):876–885, 2020. This study from the southern Appalachian Mountains, US, suggests that some pyrophilous fungi exist as mycorrhizae and endophytes when not stimulated by fire to produce fruiting structures. Return to text.
- Carroll, G., Endophytes in stems and leaves: from latent pathogen to mutualistic symbiont, Ecology 69(1):2–9, 1988. Return to text.




Readers’ comments
Comments are automatically closed 14 days after publication.