ATP synthase: majestic molecular machine made by a mastermind
First published on home page 22 Nov 2010; last update 7 Sep 2023
(Adapted from Kanehisa Laboratories, <genome.jp/kegg>)
Life depends on an incredible enzyme called ATP synthase, the world’s tiniest rotary motor.1 This tiny protein complex makes an energy-rich compound, ATP (adenosine triphosphate). Each of the human body’s 14 trillion cells performs this reaction about a million times per minute. Over half a body weight of ATP is made and consumed every day!
All living things need to make ATP, often called the “energy currency of life”. ATP is a small molecule with a big job: to provide immediately usable energy for cellular machines. ATP-driven protein machines power almost everything that goes on inside living cells, including manufacturing DNA, RNA, and proteins, clean-up of debris, and transporting chemicals into, out of, and within cells. Other fuel sources will not power these cellular protein machines for the same reasons that oil, wind, or sunlight will not power a gasoline engine.
Logical thinking about an automobile engine leads us to think that only a clever person (with mind and will) could make a machine that converts energy from one form to another for the purpose of moving a car.2 The machine shows orderly, non-random proportions and clever use of interdependent parts that are the right size, shape and strength to work together for an overall purpose. The same inference from machine back to maker is validly inferred from machines found in “nature” back to their Creator.3 Everyone knows that a painting comes from a painter, because the painting shows specified complexity, or a complex and recognizable pattern that is not a property of the paint. That is, the paint molecules do not spontaneously organize themselves into a portrait of Mona Lisa, for example.4

Figure 2: Ribbon diagram of a top view of the head portion of ATP synthase, called “F1-ATPase”. It has six protein subunits, and consists of three active sites, where three ATP molecules are formed for each full rotation of the axle. The very top end of the axle is just visible, leaning against the upper right inside wall of F1. Cellular machinery constructs the head, after which it self-assembles onto the base.
(rcsb.org/pdb/explore/images.do?structureId=2F43)
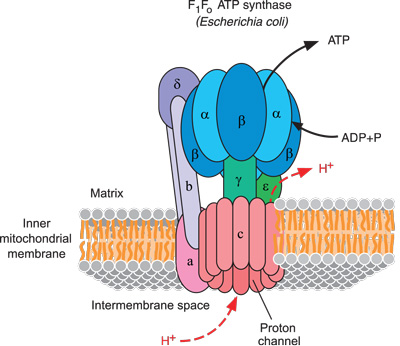
ATP synthase occurs on the inner membranes of bacterial cells, and the innermost membranes of both mitochondria and chloroplasts, which are membrane-bound structures inside animal and plant cells (see figure 1).
ATP synthase manufactures ATP from two smaller chemicals, ADP and phosphate. ATP synthase is so small that it is able to manipulate these tiny molecules, one at a time. ATP synthase must convert some other form of energy into new ATPs. This energy is in the form of a hydrogen ion (H⁺) gradient, which is generated by a different whole protein system to ATP synthase.5 Hydrogen ions pour through ATP synthase like wind through a windmill. This comprises a positively charged electric current, in contrast to our electric motors, which use a negative current of electrons.
ATP synthase is a complex engine and pictures are necessary to describe it. Scientists use clever techniques to resolve the exact locations of each of many thousands of atoms that comprise large molecules like ATP synthase.6 This protein complex contains at least 29 separately manufactured subunits that fit together into two main portions: the head (figure 2) and the base (figure 3).7 The base is anchored to a flat membrane (figure 1) like a button on a shirt (except that buttons are fixed in one place, whereas ATP synthase can migrate anywhere on the plane of its membrane). The head of ATP synthase forms a tube (figure 2). It comprises six units, in three pairs. These form three sets of docking stations, each one of which will hold an ADP and a phosphate. ATP synthase includes a stator (stationary part), which arcs around the outside of the structure to help anchor the head to the base (figure 1).

(rcsb.org/pdb/explore/images.do?structureId=2CYD)
Now we can look at the amazing, efficient way that this marvelous micro-machine works. Notice in figure 1 a helical axle labeled “γ” in the middle of the ATP synthase. This axle runs through the center of both the head and base of ATP synthase like a pencil inside a cardboard toilet paper tube.
Here is the “magic”: When a stream of tiny hydrogen ions (protons) flows through the base and out the side of ATP synthase, passing across the membrane, they force the axle and base to spin.8 The stiff central axle pushes against the inside walls of the six head proteins, which become slightly deformed and reformed alternately.9 You body has trillions of cells with many thousands of these machines spinning at over 9,000 rpm.10
The spinning axle causes squeezing motions of the head so as to align an ADP next to a phosphate, forming ATP … in bucket loads. Many other cellular protein machines use ATP, breaking it down to ADP and phosphate again. This is then recycled back into ATP by ATP synthase. A 2021 paper says, “FₒF₁ ATP synthase is a ~100% efficient molecular machine for energy conversion in biology, and holds great lessons for man-made energy technology and nanotechnology.”11

This motor is incredibly high-tech design in nano-size.
Evolutionary scientists have suggested that the head portion of ATP synthase evolved from a class of proteins used to unwind DNA during DNA replication.12
However, how could ATP synthase “evolve” from something that needs ATP, manufactured by ATP synthase, to function? This bizarre suggestion underlines the role of our beliefs in how we interpret origins. Evolutionists are often driven by a bias which they do not admit: methodological naturalism. This is the assumption that the processes which explain the operation of phenomena are all we can use to describe the origin of those phenomena. This philosophy excludes God, by decree (not because of science or reason).13
Creation scientists, looking at the same ATP synthase “phenomenon” also have a bias: supernatural origins are possible in a theistic universe. The big question is: whose bias is correct? I submit that a creation bias is clearly true because it makes sense according to the principles of causality as well as the revealed Word of the Creator Himself.
We might also consider that ATP synthase is made by processes that all need ATP—such as the unwinding of the DNA helix with helicase to allow transcription and then translation of the coded information into the proteins that make up ATP synthase. And manufacture of the 100 enzymes/machines needed to achieve this needs ATP! And making the membranes in which ATP synthase sits needs ATP, but without the membranes it would not work. This is a really vicious circle for evolutionists to explain.
What about the characteristics of the One who designed the amazing abilities of the ATP synthase nano-motor? Keep in mind that the smaller a machine is, the more ingenious the effort needed to build it.
ATP synthase speaks of wisdom, intelligence, capability, or rationality in its creator, some of the exact attributes of God as revealed in the Bible! When we investigate His handiwork, we are both obeying His command in Genesis 1:28 to do the work necessary to “subdue the earth”, and we have even more reason to praise and enjoy Him for His providence and genius.
The 20-nanometer motor (height), ATP synthase (one nanometer is one thousand-millionth of a metre). These rotary motors in the membranes of mitochondria (the cell’s power houses) turn in response to proton flow (a positive electric current). Rotation of the motor converts ADP molecules plus phosphate into the cell’s fuel, ATP.
References and notes
- For more details see Sarfati, J., Design in living organisms (motors: ATP synthase), Journal of Creation 12(1):3–5, 1998; <creation.com/motor>. Return to text.
- Using the principle of cause and effect: that something that has a beginning has a sufficient cause. Return to text.
- In philosophy, this is the teleological argument for God. Return to text.
- How can you tell if something is designed? Isn’t that pretty subjective? Access Research Network, <arn.org/idfaq/How can you tell if something is designed.htm>. Return to text.
- The energy to create the hydrogen ion gradient comes from photosynthesis or respiration of sugars. Return to text.
- These include Fluorescence Resonance Energy Transfer, Electron Microscopy, Scanning Tunneling Microscopy, and especially Nuclear Magnetic Resonance Spectroscopy and X-Ray Crystallography. Images of proteins generated by these techniques are stored online in several places, including the Protein Data Bank. Return to text.
- Stock, D., Leslie, A., Walker, J., Molecular architecture of the rotary motor in ATP synthase, Science 286(5445):1700–1705, 1999. Return to text.
- Seelert, H., et al., Proton-powered turbine of a plant motor, Nature 405(6785):418–419, 2000. Return to text.
- DNAtube: Scientific video site, <dnatu be.com/video/1197/ATP-Synthase–Part-I>. Return to text.
- ATP Synthase, <mrc-mbu.cam.ac.uk/research/atp-synthase>. However, only about 10% of human cells have mitochondria; about 90% do not, mainly red blood cells and platelets. Return to text.
- Hou, R. and Wang, Z., Thermodynamic marking of FₒF₁- ATP synthase, Biochimica et Biophysica Acta (BBA)—Bioenergetics 1862(4):148369, 1 Apr 2021. The 100% efficiency applies to conversion of rotational kinetic energy into the chemical potential energy of ATP, or the reverse conversion. Many modern biochemistry textbooks claim 55–60% efficiency. Taking into account the energy inputs and outputs of all the metabolic steps, the efficiency is 40–41% (Nath, S., The thermodynamic efficiency of ATP synthesis in oxidative phosphorylation, Biophysical Chemistry 219:69–74, Dec 2016). But this is still much higher than most man-made motors. Return to text.
- Evolution of the F₁-ATPase, <uiuc.edu/crofts/bioph354/Evol_F1.html>. Here, Professor Antony Crofts of the University of Illinois concludes from his comparison of the head region of ATP synthase to a hexameric helicase enzyme that “… homologous tertiary structure [somewhat similar shape] strongly suggest that these two types evolved from a common ancestor …”. But it is only a “strong suggestion” when supernatural origins are ruled out by definition! And though Crofts highlights the similarities between these enzyme systems, the differences are insurmountable by naturalistic origins hypotheses. Return to text.
- See Wieland, C., The rules of the game, Creation 11(1):47–50, 1988; <creation.com/rules>. Return to text.





Readers’ comments
Comments are automatically closed 14 days after publication.