Den of ape-men or chambers of the sickly?
An update on Homo naledi
On Tuesday 9 May 2017, the second live streaming instalment of the Homo naledi saga occurred, broadcast from Wits University, Johannesburg, South Africa. Lee Berger, Paul Dirks and John Hawks took turns in presenting the latest findings regarding the so-called hominin (or hominid) fossils from the Rising Star cave system.

They were also first authors on three new papers on the topic, published on the same day.1,2,3 Paleoanthropologist Lee Berger, of Wits University, is the leader of the Rising Star research team. A similar live streaming event occurred on 10 September 2015, introducing the alleged ‘ape-man’ species Homo naledi to the world, accompanied by the initial Homo naledi publications.4,5
Of particular interest was the paper by Hawks et al. dealing with the newly unveiled Homo naledi fossils.2 In this paper we learn about fossils found in a second chamber of the Rising Star cave system, the Lesedi Chamber (locality UW 102). The Lesedi Chamber is said to have produced 131 fossil specimens, thought to represent a minimum of three individuals (two adults and a juvenile). This is a much smaller number than was earlier reported on for the Dinaledi Chamber (locality UW 101), of the same Rising Star cave system, said to so far have produced a minimum of 15 individuals, represented by more than 1550 fossil specimens.
The Lesedi Chamber is stated as being about 30 m (100 ft) below the surface, there being no direct route between it and the Dinaledi Chamber. The two chambers are said to be about 60 m (200 ft) apart in a straight line, with the shortest traversable route between them being approximately 145 m (475 ft). Excavations in the Lesedi Chamber were carried out in three areas, with most of the fossil specimens coming from area UW 102a, including the partial LES1 skeleton, nicknamed ‘Neo’. The LES1 skeleton includes a relatively complete skull, with cranial capacity of about 610 cc (cubic centimetres). Examination of the fossils indicate they are morphologically consistent with the previously described Homo naledi fossils. Evidence is said to be consistent with the bodies arriving intact in the Lesedi Chamber, there being no signs that the remains had been exposed to the surface environment. The area excavated in the Lesedi Chamber is said to be small, so it is likely that more fossils will be discovered. No tools have been found in either of the chambers so far.
According to Hawks the research team has attempted to recover DNA from bones in the Dinaledi Chamber, but were not successful in this.6 Currently there is no age date for the fossils in the Lesedi Chamber, but there is now a date for the fossils from the Dinaledi Chamber. According to Hawks six different methods were used to date the age of the fossils, but the “most valuable of these were electron spin resonance (ESR) dating, and uranium-thorium (U-Th) dating”.7 Dirks is first author of the paper on the dating of the Homo naledi fossils from the Dinaledi Chamber of the Rising Star cave system. In the paper, Dirks et al., by “combining the US-ESR maximum age estimate obtained from the teeth, with the U-Th age for the oldest flowstone overlying Homo naledi fossils”, concluded that the most likely age of the fossils is between 236,000 and 335,000 years old.8
The findings of the new Hawks et al. paper, as well as other recent developments and publications about Homo naledi, such as the age date (see discussion), will be examined here, in order to re-evaluate and update aspects of my previous published works on Homo naledi.9,10 The focus of the morphology of Homo naledi examined below are some of the features considered most informative of its taxonomic position.
Vertebrae
In the initial Berger et al. paper the description of the vertebrae was consistent with Homo naledi being human, with the transverse processes of the Homo naledi T10 and T11 vertebrae described as most similar to Neandertals in morphology.11 A follow-up paper on the vertebrae and ribs of Homo naledi, by Williams et al., did not contradict this earlier assessment.12 They did note, as did the original Berger et al. paper, that the vertebrae of Homo naledi were small in overall size, but that the spinal canal area was relatively large.13
The new Hawks et al. paper states that the Dinaledi Chamber and Lesedi Chamber two fossil vertebrae pairs (T10 and T11) “are comparable in size, but the Lesedi vertebrae clearly belong to a larger, more robust (presumed male) individual”.14 However, they did report that the orientation of the transverse processes were lower (i.e., more posterior) in the Homo naledi T10 and T11 Lesedi vertebrae compared to the same T10 and T11 Dinaledi Chamber vertebrae, and in the case of the Lesedi T10 vertebra it was lower than in any other ‘hominin’, whereas the Dinaledi T10 was “similar to the Neandertal value and extremely low compared to that for modern humans”.15 What this all means is unclear (e.g., is there pathology?), but overall the vertebrae of Homo naledi are consistent with it being human, albeit most similar to Neandertals (‘robust’ humans) in regards to vertebrae.
Rib cage
In the initial Berger et al. paper the rib cage of Homo naledi is described as “wide distally like Au. afarensis”, and elsewhere in the paper the evidence from the ribs is assessed as “suggesting that the thorax was pyramidal in shape”.16 In the team’s follow-up paper on the vertebrae and ribs of Homo naledi they “speculate that the apparent lack of rib torsion in this hominin thus suggests a wide lower thorax morphology, perhaps coupled with a cranially convergent upper thorax that is dissimilar to that of modern humans and perhaps Neandertals and other members of the genus Homo”.17
However, the suggestion of a “narrow upper thorax” is speculative as the authors admit that the “proximal upper rib ends in H. naledi are too incomplete to allow reconstruction of their curvature”.13 In the new Hawks et al. paper additional rib fragments were found, but only a nearly complete right first rib (UW 102a-250) added much in terms of useful information, the rib being described as “more complete than the Dinaledi first rib fragments, and is similar in morphology in the overlapping regions”.15 The rib is also stated as being “similar in morphology and size to known australopith first ribs”.15

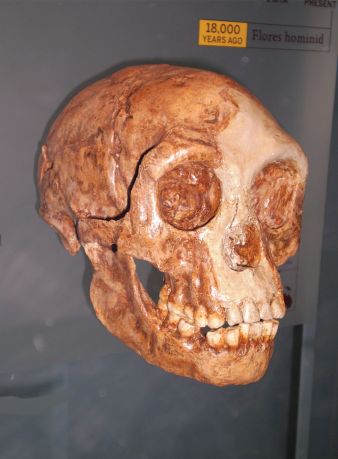
Credit: Creative commons. Dirks et al., ref. 5, p. 6.
The Homo erectus Nariokotome boy (KNM-WT 15000) is described as having a barrel-shaped thorax, like us.18 Before 2001, the Neandertal rib cage had been illustrated in textbooks to look like a “barrel-shaped human model”.19 However, in 2001 an assembled entire Neandertal skeleton (consisting of fossil elements from several different sites) “boasted a conical thorax that tapered upward from the broad pelvis to a narrow top, giving it an incredibly distinctive look”.20 The reconstructed rib cage of Australopithecus afarensis (represented by the famous specimen Lucy AL 288-1) is described as being “shaped like a funnel, with the narrow part at the top and a wide lower region”.18
Hence, a wide distal (lower region) rib cage can, apart from being interpreted to be like Australopithecus afarensis, also be interpreted as being similar to that of the Neandertals. As Neandertals (and Homo erectus) are regarded by this author (and most creationists) as fully human, the rib cage does not preclude Homo naledi from also being human, even if its lower rib cage is broad, as suggested by the authors, as it may fall within human variation. However, simply “suggesting that the thorax was pyramidal in shape” sounds unconvincing.11 But what if it does turn out to be pyramidal in shape, as suggested by the authors, and it is different from the Neandertal thorax? Apart from belonging to an ape-like australopith creature, what other explanation is possible? A paper by Donald Ortner and Gerhard Hotz show a photo illustrating a case of hypothyroidism (e.g., cretinism) in the thorax part of the skeleton of an 80-year-old modern human male.21 In the caption it says: “Note triangular shape of thorax, indicating greatly diminished development of costae of cranial end of thorax relative to lower costae”.21 In the 12 cases examined for skeletal growth disturbances in hypothyroidism, eight cases had disproportionate development of the rib cage, with the authors stating that “disproportionate development of the costae typically more severe in the cranial end of the thorax”.22 Hence, a pyramidal or triangular thorax in Homo naledi could be evidence that one is dealing with a developmental pathology, such as cretinism.
Shoulder
Humeral torsion
The initial Berger et al. paper states that the shoulder of Homo naledi “is configured with the scapula situated high and lateral on the thorax, short clavicles, and little or no torsion of the humerus”.11 Humeral torsion is an angle that “refers to the orientation of the humeral head relative to the distal end of the humerus”.23 In a follow-up paper on the upper limb of Homo naledi the reported humeral torsion value (91°) of Homo naledi in a mature humerus (UW 101-283) is well below the range of both fossil ‘hominins’ and extant ‘hominoids’.24 The humeral torsion value of an immature Homo naledi humerus (UW 101-948) is also given (105°).25 In the Hawks et al. paper the newly described proximal humeri fragments from the Lesedi Chamber are said to “have morphology consistent with the Dinaledi Chamber collection of H. naledi”, but humeral torsion “cannot be assessed directly in the fragments from the Lesedi Chamber”.26

Low humeral torsion (111.5°) is also present in the immature humerus of the Homo erectus Nariokotome boy (KNM-WT 15000),27 and the Homo erectus Dmanisi mature (D4507: 110.0°) and immature (D2680: 104.0°) humeri.28 For comparison, Feuerriegel et al. list mature humeral torsion values for a range of species, in addition to the above, including Homo sapiens (144.1°), Pan troglodytes (139.0°), Homo floresiensis (LB1: 115.0°), Australopithecus sediba (MH2: 117.0°) and Australopithecus afarensis (AL 288-1: 124.0°).25
A paper by Eckhardt et al. commented on the low initial estimate of humeral torsion (110°) in the Homo floresiensis LB1 specimen (which had subsequently been revised to a higher value of 115° or 120°, within the range of “extant small-bodied humans”), stating:
“The reported humeral torsion, being outside the range of modern humans, was held to constitute another uniqueness of H. floresiensis. However, this degree of torsion also is outside those of all large-bodied apes and hominins. H. floresiensis, as a member of the genus Homo, should have humeral torsion within the hominid (large apes plus humans) range, were it a healthy normal individual, so its reported value was more likely to be diagnostic of pathology than uniqueness.”29
It should be noted that the initial LB1 humeral torsion value (110°) appears to have first been published in 2005,30 which was before the publication of the Dmanisi Homo erectus humeral torsion values in 2007,28 and so at the time a humeral torsion of 110° appears to have been the lowest for any ‘hominin’. The interesting question now is, if some evolutionists considered a low humeral torsion value of 110° to be “more likely to be diagnostic of pathology than uniqueness”, what does that say about the much lower Homo naledi humeral torsion value of 91°?
Clavicle and scapula
Concerning the short clavicle of Homo naledi, a relatively short clavicle has also been reported for the Homo erectus Nariokotome boy (KNM-WT 15000),31 and so a short clavicle is not inconsistent with Homo naledi being human. As for the suggestion that the scapula is situated high on the thorax in Homo naledi, this is also a possible interpretation of the Nariokotome boy specimen.32 However, further analysis by Feuerriegel et al. led them to conclude that the Homo naledi Dinaledi clavicular remains were similar in gross morphology to the clavicular specimens attributed to Australopithecus sediba (MH2), Homo habili s (OH 48), and Pan, “suggesting that the H. naledi shoulder position was more superior than in modern humans or KNM-WT 15000”.33 In the Hawks et al. paper the newly described Lesedi Chamber claviculae are said to be consistent with the earlier described Dinaledi Chamber clavicular material, and so does not change the above picture.34
Scapulae from australopithecines such as Australopithecus afarensis specimen AL 288-1 (better known as Lucy) and Australopithecus africanus specimen Sts 7, as well as the great apes, differ from that of modern human scapulae in having a more cranially oriented glenoid fossa (cavity), indicating habitual use of the arm in an elevated position “that would be common during climbing behavior”,35 such as suspensory arm-swinging.36 Studies of the more complete right scapula of the Nariokotome boy indicate that the glenoid fossa in Homo erectus was not cranially oriented; although a Dmanisi Homo erectus scapular fragment was more cranially oriented than that of the Nariokotome boy, it was still within the modern human range.37
The recent Hawks et al. paper mentions that “several fragments of scapula” were found in the UW 102a area of the Lesedi Chamber,38 but as these appear not to be elaborated on further in the paper, except listed in Table 1, it is presumed the fragments did not contribute much extra to the understanding of Homo naledi. The earlier follow-up paper analyzing the shoulder, by Feuerriegel et al. reported that “Homo naledi had a scapula with a markedly cranially-oriented glenoid”,39 being as cranially oriented as gibbons (hylobates), and more cranial than the great apes (chimpanzees, orangutans, and gorillas), modern humans (Homo sapiens), Australopithecus afarensis (AL 288-1), Australopithecus africanus (Sts 7), Australopithecus sediba (MH2), and Homo erectus (KNM-WT 15000).40 The authors conclude that:
“Homo naledi had a scapula with a markedly cranially-oriented glenoid, a humerus with extremely low torsion, and an australopith-like clavicle. These traits indicate that the H. naledi scapula was situated superiorly and laterally on the thorax. This shoulder girdle configuration is more similar to that of Australopithecus and distinct from that of modern humans, whose scapulae are positioned low and dorsally about the thorax. Although early Homo erectus maintains many primitive clavicular and humeral features, its derived scapular morphology suggests a loss of climbing adaptations.” 39
From an evolutionary point of view, Homo naledi could not have been a transitional form between the australopithecines and a later species of Homo, as its shoulder was even more cranially-oriented then its hypothetical australopithecine ancestor. Can Homo naledi’s shoulder make more sense from a creation point of view? According to Williams et al., “a strongly cranially-oriented glenoid and an elevated clavicle and shoulder are suggestive of a narrow upper thorax”.13 If true, this may suggest some sort of developmental linkage between shoulder girdle position and width of the upper thorax. Hence, if someone had a narrow upper thorax due to cretinism, as discussed in the rib cage section above, would that then conversely suggest the person would likely have a cranially-oriented glenoid and elevated (superior) shoulder? Perhaps, but then it would have nothing to do with evolution, but rather a developmental pathology.
Hand and wrist
The new Hawks et al. paper reports finding four new hand and wrist elements in the Lesedi Chamber 102a area, but as they are stated as being “qualitatively similar in overall shape to that described for H. naledi”, although “absolutely larger in most of their overall dimensions”, they do not contribute anything new of significance to the discussion of the Homo naledi hand and wrist.41 In the initial paper by Berger et al. it is stated that the hand of Homo naledi “shares many derived features of modern humans and Neandertals in the thumb, wrist, and palm, but has relatively long and markedly curved fingers”.11 A later follow-up publication on the hand of Homo naledi by Kivell et al. essentially told the same story as the initial paper, stating:
“the wrist and palm are generally most similar to those of Neandertals and modern humans, while the fingers are more curved than some australopiths. This distinctive mosaic of morphology has yet to be observed in any other hominin taxon and suggests the use of the hand for arboreal locomotion in combination with forceful precision manipulation typically used during tool-related behaviours.”42
There appears to be something very strange about the curvature of Homo naledi’s fingers, and that is the high degree of curvature of not just the proximal phalanges (PPs), but also the intermediate phalanges (IPs). At face value the fingers of Homo naledi appear better suited to climbing than chimpanzees, as the PPs are about the same curvature, but Homo naledi’s IPs are considerably more curved than those of chimpanzees and australopithecines, the median value even higher than orangutans.43 According to the authors, “extant apes and most fossil hominins, such as A. afarensis and OH7, generally have more strongly curved PPs and comparatively straight IPs”.44 Yet, other aspects of Homo naledi’s hand, such as the “thumb, wrist, and palm bones all look remarkably modern”.45 Hence, most of the Homo naledi hand is human-like, except for the markedly curved fingers, stated as “a clear functional indication that its fingers experienced high loads during grasping required for climbing or suspensory locomotion”.44
In regards to phalangeal curvature, it should be noted that “degree of longitudinal curvature is strongly correlated with the degree of arboreal locomotion across primates, with climbing and, especially, suspensory taxa showing much stronger curvature than terrestrial quadrupedal or bipedal taxa”.46 Also, changes in phalangeal curvature appear to be associated with functionality (i.e., locomotion) during ontogeny, “such that more arboreal juveniles have more strongly curved phalanges than their more terrestrial adult counterparts”.46 A study on the biomechanics of phalangeal curvature concluded that “the strain differences between curved and straight phalanges illustrated here support the common assertion that phalangeal shaft curvature is related to the strains associated with arboreal and especially suspensory activity … , and may underlie changes in curvature during ontogeny in response to changes in mechanical environments of arboreal and terrestrial supports”.47
Homo naledi’s hand does not make sense in an evolutionary scenario because, if Homo naledi was transitional between the australopithecines and a later species of Homo, then functionally (as indicated by finger curvature) it appears that Homo naledi was even better suited to an arboreal lifestyle than its hypothetical australopithecine ancestor, when it should be less so. As with the markedly cranially-oriented glenoid fossa orientation of the shoulder, as well as the extremely low humeral torsion, it is unlikely that the high degree of phalangeal curvature exhibited by Homo naledi can be explained by normal human variation, if indeed the hand is from a human.
It is interesting regarding Homo floresiensis that its proximal phalanges are said to be “curved to a similar degree as in Au. afarensis”.46 The author appears to be referring to a bone that belongs to the LB6 Homo floresiensis individual. The authors of the publication that analyzed this bone commented on the curvature of the shaft that “LB6/8 falls at the extreme upper end of the human range and overlaps with gorillas. It is similar in this respect to A.L. 333w-4, an Australopithecus afarensis specimen”.48 The proximal manual phalanges of the Homo floresiensis LB1 individual appear to be too incomplete to make a conclusive judgement on curvature.49 No information appears to be given on the curvature of the intermediate manual phalanges of the LB1 and LB6 Homo floresiensis individuals.50
The species designation of Homo floresiensis has been controversial, as it has been argued by some evolutionists that it instead consists of individuals, such as LB1 and LB6, that “are, most likely, endemic cretins from a population of unaffected Homo sapiens”.51 Hence, did the Homo naledi individuals suffer from cretinism, in a similar way that individuals from the Homo floresiensis species possibly did, with the curved fingers related to cretinism or associated conditions?
Pelvis
The recent Hawks et al. paper describes a new ilium fragment (UW 102a-138) from an immature individual, and its morphology is said to be comparable to that of a Dinaledi Chamber juvenile Homo naledi ilium fragment (UW 101-486), “and thus consistent with the morphology seen in H. naledi”.52 However, because of poor preservation the ilium fragment “lacks the diagnostic characters that could differentiate it clearly from ilium fragments from other hominin species”, and so it does not add much to the discussion of Homo naledi.52
Concerning the adult pelvic material of Homo naledi from the Dinaledi Chamber, the authors state it “is notable in combining an Au. Afarensis-like degree of iliac flare, a weak and anteriorly placed iliac pillar, and a narrow tuberoacetabular sulcus on the ischium”.52 This is consistent with the initial Berger et al. paper that said the pelvis of Homo naledi “appears to be flared markedly like that of Au. Afarensis”.53
There are pelvic bones attributed to Homo erectus that are described as having “broad, laterally flaring ilia”, including the Gona specimen (BSN49/P27), OH 28 and KNM-ER 3228.54 According to Gruss the “pelvis of H. erectus, while broad compared with modern humans, was narrower relative to body height than in the australopithecines”.55 As opposed to being markedly laterally flared, in modern humans the iliac blades curve or wrap around the sides of the body considerably more. The australopithecine ilium has been described as “excessively broad”, such that the “breadth of the human iliac blade is actually intermediate between those of the chimp and of Australopithecus”.56A presentation of the pelvic features by VanSickle et al. reported that the angle of lateral iliac flare on the best preserved pelvic fossil (UW 101-1100) in the Homo naledi sample was:
“identical to that seen in Australopithecus fossils like Lucy and Sts 14. It is such a wide angle that there is no way to reconstruct the Homo naledi hip to make it look not-flared. This extreme amount of flare is a primitive hominin feature that is not found in other Homo pelvic remains, even though fossil Homo pelves have been described as being more flared than modern humans”.57
The authors note that it is possible the Gona pelvis also has similar extreme amount of flare, like the Homo naledi pelvis, but that it may not matter as there is debate about whether the pelvis is a species of Australopithecus rather than Homo erectus.57 Hawks states that “the pelvis of H. naledi exhibits a short, flared ilium unlike those known for H. erectus, including the Gona pelvic specimen”.58 Hence, it appears the extreme lateral iliac flaring observed in the Homo naledi pelvis is outside the range of Homo erectus. Interestingly, one of the features noted in cretinism is lateral flaring of the ilium of the pelvis.59 Similar to the description of the Homo naledi pelvis, it has been stated regarding the pelvis of the Homo floresiensis type specimen (LB1) that its “marked degree of lateral iliac flaring recalls that seen in australopithecines such as “Lucy” (AL 288-1)”.60 As already mentioned, some evolutionists believe individuals from Homo floresiensis were actually pathological humans, with cretinism a plausible explanation.51
Foot and ankle
There appears to be no mention of any foot or ankle specimens from the Lesedi Chamber, so nothing new is added about Homo naledi in this regard. In the initial paper on Homo naledi, the Berger team stated that “the foot and ankle are particularly human in their configuration”.53 Essentially the only traits of its foot regarded as “primitive” are evidence “suggestive of a lower arched foot”11 and “slightly more curved toe bones”.61 Paleontologist Will Harcourt-Smith, lead author on a publication on the Homo naledi foot62 that essentially told the same story as the initial paper, states it “is essentially the foot of a modern human, but subtly different”.61 Paleoanthropologist Dan Lieberman is quoted as saying: “The foot is indeed strikingly modern … and suggests it walked and possibly ran much like modern humans.”63
According to evolutionary experts: “All primates possess a transverse arch, but only humans have a longitudinal arch making non-human primates anatomically and functionally flat-footed.”64 The longitudinal arch is a structure involved in storing elastic energy and it “maintains the structural rigor of the foot during the push-off stage of bipedal locomotion”.64 As for the lower arched foot, the Berger group state that Homo naledi “likely had minimally developed longitudinal foot arches (i.e., flatter feet), which is uncommon (but not unknown) in living people”.65 This “relatively low medial longitudinal arch” interpretation appears to be based on one foot (Foot 1).66 However, flatfoot is a frequently encountered pathology in both pediatric67 and adult68 human populations, and is not regarded as a ‘primitive’ condition in modern humans, and so its possible presence in the foot of Homo naledi is probably not significant. It is interesting to note that, according to Jungers et al., in Homo floresiensis the big toe (hallux) was fully adducted (in line with the rest of the foot), but a medial longitudinal arch was suspected to be absent.60 Hence, Homo floresiensis probably had much flatter feet than Homo naledi.
The Berger team mentions human-like features of Homo naledi, for example, that their “big toes were in-line with the rest of the foot, unlike the grasping, opposable big toe in chimps”, but also mentions that their “toes were also slightly curved— not as much as a chimp’s toes— but more than in humans”.65 The range of curvature in the pedal proximal phalanges of Homo naledi appear to overlap considerably with Homo sapiens, so this finding is probably not that significant,69 although it is a little bit odd in that it does not appear to reflect any functionality. To be used effectively for climbing in trees the feet of Homo naledi would need to have a grasping, opposable big toe as chimpanzees do, but Homo naledi’s big toe was in line with the rest of the foot, like in humans. It is interesting that the toe bones of Homo floresiensis are also said to be slightly curved (i.e., the proximal pedal phalanges).60 As already mentioned, Homo floresiensis is possibly associated with cretinism.
Thigh bone (femur) and shinbone (tibia)
Based on a tibia (UW 101-484), the stature of one Homo naledi individual was estimated to be just under 1.5 m (4 ft 9 ins), whereas body mass was estimated, from eight femur specimens, to vary from about 40 kg (88 lbs) to 56 kg (123 lbs); with estimates of both stature and body mass comparable to small-bodied humans.70 It is stated that locomotor “traits shared with Homo include the absolutely long lower limb”,71 which is consistent with Homo naledi being human-like. Homo naledi is said to possess a valgus knee72 (angling inward of the femur making the knees closer together), a characteristic of humans that allows efficient bipedalism.
In the initial Berger et al. paper Homo naledi’s femoral neck is stated as being anteroposteriorly compressed (i.e., femoral necks that are narrow anteroposteriorly relative to superoinferiorly),73 a feature generally considered an “archaic morphology”,74 as it is considered typical of the australopithecines, but not in modern humans or femora attributed to Homo erectus.75 Whilst as a group the femoral neck of australopithecines are statistically anteroposteriorly compressed compared to modern humans, the data from Marchi et al. indicate some overlap between the two groups.76 Although this follow-up paper concurred with the initial Berger et al. paper that one of the “primitive” traits Homo naledi shared with the australopiths included an anteroposteriorly compressed femoral neck,77 a look at the data table shows that all the Homo naledi femurs were within the Homo sapiens range for this feature, and the two Homo erectus femora fall within the Homo naledi range.76 Hence, this is not an “archaic” morphology or “primitive” trait that supports assignment of Homo naledi fossils to a new species of ‘ape-man’.
The new Hawks et al. paper describes some new femoral fragments from the Lesedi Chamber that are mostly consistent with the previous described femoral morphology of Homo naledi from the Dinaledi Chamber.78 There appears to be one right proximal femur (UW 102a-001) and a left partial femur (made up of fragments UW 102a-003 and UW 102a-004, believed to represent the same femur), but despite the similarity in size between the overlapping regions of the left proximal (UW 102a-003) and right proximal (UW 102a-001) femoral fragments, they “contrast in several anatomical details”.79 According to Hawks et al. the two femoral fragments “are different in muscle attachments and diaphyseal morphology, to the extent that would represent unusual asymmetry in a single individual”.80 What is interesting is that the authors believe the fossil materials from the 102a area of the Lesedi Chamber “represent a minimum of two adult individuals and one immature individual”, but the “inference of two adults is based upon the morphological incongruence” of the left and right femoral elements, as “no adult element is clearly duplicated in the collection”.78 The authors state that on “the basis of the non-duplication of elements, it seems likely that if there is a second individual, this second individual is represented by only a small number of elements, possibly just the femur”.80
Rather than invent a second adult individual to explain away difficult evidence, it seems to me that the most parsimonious explanation is that there may only be one adult represented in the 102a area of the Lesedi Chamber, and that the morphological incongruence of the left and right femoral fragments is possibly due to pathology in the individual. Limb asymmetries are a possible manifestation of hypothyroidism (e.g., cretinism).81 For example, Ortner and Hotz show a photo illustrating abnormal medial rotation of the left leg in a 67-year-old modern human female that had hypothyroidism.21 They do note that the “variability seen in the skeletal manifestations of hypothyroidism ranges from no effect at all to severe dwarfism. The skeletal abnormalities tend to be bilateral, but within a single patient some bone groups may be severely involved, and while [sic] others are relatively normal”.82
Skull
In the initial Berger et al. paper the authors state that the “morphology of the cranium, mandible, and dentition is mostly consistent with the genus Homo, but the brain size of H. naledi is within the range of Australopithecus”.53 In Chris Stringer’s accompanying eLife article, Homo naledi is labelled as having a “relatively high and thin skull” and small teeth, whereas Homo erectus is labelled as having a “relatively low and thick skull” and large teeth, and both the upper and lower portion of the occipital bone is angled (not rounded as in modern humans), with a transverse torus running across the angulated region.83 The Berger paper states that “compared to samples of H. habilis, H. rudolfensis, and H. erectus, the teeth of H. naledi are comparatively quite small, similar in dimensions to much later samples of Homo”.84 Having small teeth is a feature of modern humans, as is having a high and thin skull. Also, the cranial vault of Homo naledi is described as having only slight post-orbital constriction, the mandibular dental arcade as parabolic in shape, and the mandibular corpus (body) as being relatively gracile.85 These features of the skull do not align it with the australopithecines, but rather with humans, although the skull of Homo naledi is not that of an anatomically modern human.
When the Homo erectus Dmanisi Skull 5 was revealed in 2013,86 one of the big surprises was the implication of this find on the variability of Homo erectus, at least of the skull, with the morphological variation considerable.87 Is the skull of Homo naledi really that different? According to Tim White the Homo naledi fossils “are a small, primitive H. erectus”.88 John Hawks responded to White’s assessment by saying “H. naledi does not have the elongated, low cranium of H. erectus”.58 An expanded study by Laird et al. on the skulls of Homo naledi, based on the Dinaledi Chamber material, came to essentially the same conclusion as the initial Berger et al. study, that is, that the skulls were a morphologically homogeneous sample and belonged in the genus Homo, but did not fit into any existing taxa; it was noted, though, that Homo naledi shared a number of characters with Homo erectus, in particular KNM-ER 427000 and the Dmanisi sample (e.g., D4500 and D2700).89 Using geometric morphometric approaches to characterize cranio-mandibular shape, the results presented by Schroeder et al. were also “consistent with the initial descriptive diagnosis of this species”, although they did note that the Homo naledi crania had “close affiliations to H. erectus”, with Homo naledi “most closely associated with African/Dmanisi H. erectus”.90
One of the most exciting things found in the Lesedi Chamber was a relatively complete Homo naledi skull, which formed part of the LES1 skeleton. The anatomy of the LES1 cranium is said to reinforce “in most respects” the conclusions of the above-mentioned studies, such as Schroeder et al. and Laird et al., as well as a study by Rightmire et al.,91 that addressed the morphology of Homo naledi crania in comparison to the Dmanisi crania.92 According to Hawks et al. most of the features of the LES1 vault are characteristic of Homo naledi from the Dinaledi Chamber.93 According to Ann Gibbons:
“First announced in 2015, H. naledi was a puzzle from the start. Fossils from 15 individuals, including fragile parts of the face that are preserved in the new skull, show that the species combines primitive traits such as a small brain, flat midface, and curving fingers with more modern-looking features in its teeth, jaw, thumb, wrist, and foot. Berger’s team put it in our genus, Homo.”94
The LES1 skull preserved parts of the face that were not available from the Dinaledi fossil material and, as Gibbons states above, what it reveals is that Homo naledi appears to have had a flat midface, a trait said to be “primitive”. What is interesting is that a flat midface is also a trait associated with cretinism.95 Hence, this is one more indicator that perhaps what we are dealing with in Homo naledi is some sort of developmental pathology, but not in modern humans, rather in humans broadly categorized as Homo erectus and Homo heidelbergensis (or ‘archaic’ Homo sapiens). I categorize these and all other non-modern fossil humans, including Neanderthals, as ‘robust’ humans.
There is indisputable evidence that the morphology of skulls classified by evolutionists as Homo erectus varies considerably, a point illustrated by Schwartz et al.96 Regardless of whether Homo naledi is classified as Homo erectus or not, the form of the Homo naledi skull appears to be within human variation (here human variation encompasses the combined range of both modern and robust humans).
Perhaps the most astonishing aspect of Homo naledi is its small cranial capacity.97 Homo naledi was initially characterized as having “body mass and stature similar to small-bodied human populations but a small endocranial volume similar to australopiths”.98 Estimates of cranial capacity have produced very small values for both composite crania from the Dinaledi Chamber, DH3/DH4 (465 cc) and DH1/DH2 (560 cc).99 The new LES1 cranium from the Lesedi Chamber, with a cranial capacity of about 610 cc, is slightly larger than the Homo naledi crania from the Dinaledi Chamber.100
Before Homo naledi, the smallest estimate of cranial capacity of a Homo erectus skull from Africa, at 691 cc, was KNM-ER 42700, believed to be of “a young adult or a late subadult”.101 Outside Africa, smaller Homo erectus cranial capacities have been estimated from Dmanisi, Georgia. A cranial capacity of 546 cc for the adult Dmanisi cranium D4500 is the smallest of the Dmanisi sample, with the capacities of the other four crania reported to be between 600 cc to 730 cc (D2280: 730 cc; D2282: 650-660 cc; D2700: 600 cc; D3444: 625 cc).102 Of additional interest is the LB1 Homo floresiensis cranium, estimated to be 426 cc.103 The mean cranial capacity for modern humans is about 1345 cc, but the range of modern humans able to function normally is difficult to specify, although approximately 700 cc to 2200 cc is given by expert Stephen Molnar, who comments that “there are many persons with 700 to 800 cubic centimetres”.104 One of the smallest brain sizes documented of a modern human with normal intelligence was from Daniel Lyon, a man of small stature (height of 1.55 m [5 ft 1 in]), with a brain volume of about 624 cc,105 and hence an estimated cranial capacity of 660 cc.106
The mean cranial capacity of Homo erectus is about 917 cc,107 which is considerably lower than the modern human mean of 1345 cc. That on average Homo erectus specimens had much smaller cranial capacities may reflect something intrinsic about these humans, that is, perhaps the range of what could be considered normal brain size would have been lower in Homo erectus compared to modern humans. The same argument could be applied to the Homo naledi crania, but ultimately the answer to such speculation is unknowable. However, cretinism (congenital hypothyroidism) “can reduce brain size by approximately 50%”,108 and so would be one possible explanation for the low cranial capacity observed in the Homo naledi crania.
Discussion and conclusion
The Berger team considers Homo naledi ‘primitive’ in morphology compared to Homo erectus, and so prior to obtaining an age date they maintained that “the H. naledi lineage must have existed earlier than the first occurrence of H. erectus around 1.8 Ma”.109 In 2016 a phylogenetic study by Dembo et al. reported the most likely age for Homo naledi to be 912 ka.110 However, studies such as these depend on unfounded assumptions, including assuming evolutionary relationships between fossil species and accepting dates associated with fossil specimens as valid. Also, the study in question restricted the characters used to one anatomical region, the skull (including teeth), making the findings unreliable on this measure alone.111
That the alleged age of the Homo naledi fossils was dated to be much younger than anticipated, between 236 ka and 335 ka old,8 is from an evolutionary point of view akin to the species being stranded on a dead-end limb of a trunkless tree. I do not accept the above-mentioned age dates, as radiometric dating is based on unverifiable and problematic assumptions.112,113 Often the geological ages assigned to hominid fossils by evolutionists are inconsistent, varying according to the technique used. For example, with respect to the dating of some of the Java Homo erectus fossils (including Ngandong Homo erectus), one evolutionary expert lamented concerning the age that it “all depends which radiometric method you use to assess the fossils’ age”.114 In the brief article, written by Bruce Bower in 16 April 2010, he states:
“After convincing most of their colleagues that H. erectus may have persisted on the Indonesian island of Java as recently as 30,000 years ago—late enough to have coexisted in Asia with modern humans for more than 100,000 years—anthropologists presented new analyses April 14 suggesting the fossils in question may actually predate Homo sapiens by hundreds of thousands of years.”114
Interestingly, three Homo naledi bone fragments were radiocarbon dated, providing ages of about 33.0 ka and 35.5 ka (the age of the third sample is not given), with it reported that no collagen was present in any of the bone samples analyzed.115 These age dates are considerably younger than what the researchers considered “the most parsimonious age estimate for the H. naledi fossils”, stated as “sometime between 236 ka and 335 ka”.116 The much younger radiocarbon dates are explained away by the statement: “We interpret these ages to relate to late calcite precipitation in the bones that may reflect a wet period in the cave.”115 While the researchers are to be commended for publishing the ‘anomalous’ data, the discrepancy in the age results between the different methods does little to dispel concerns that the dates obtained all depend on “which radiometric method you use to assess the fossils’ age”.
In a paper titled ‘Homo naledi and Pleistocene hominin evolution in subequatorial Africa’, Berger et al. interpret Homo naledi’s status in the light of its newly assigned “parsimonious” age dates (between 236 ka and 335 ka old, in a period called the late Middle Pleistocene).117 One of their interpretations of Homo naledi is “that the species and its branch must have existed much earlier than the Dinaledi fossils”, with the authors stating that analysis of craniodental evidence suggests “that its anatomical pattern may have been present from the earliest origin of Homo”.118 Another scenario has Homo naledi “as a sister taxon to archaic species of Homo and modern humans, closer to living humans than H. erectus … If this is true, an early H. naledi population may have been the ancestor of humans, placing H. erectus as a side branch”.118 A third option places Homo naledi “as a sister to H. erectus and larger-brained Homo including H. sapiens”.119 The authors state that this option would more likely find support if postcrania are considered in analysis, because Homo naledi “shares many derived features of the hand, foot, and lower limb with H. erectus and H. sapiens that are apparently absent from H. habilis, H. floresiensis, or Au. sediba, yet lacks several derived traits of the shoulder, trunk, and hip shared by H. erectus and H. sapiens”.119 Amazingly, one of the doyens of paleoanthropology, Bernard Wood, seems to be suggesting some sort of evolutionary reversal to explain Homo naledi, as indicated in the following two paragraphs from a New Scientist article by Colin Barras:
“Bernard Wood at the George Washington University in Washington DC thinks H. naledi branched off from other humans relatively recently and then evolved to look more primitive. “Its primitive features might be misleading,” he says. For instance, southern Africa might have been relatively isolated from the rest of the continent, says Wood. Lack of competition from other humans could have relaxed the pressure on H. naledi to grow a large brain. If the skeleton no longer had to bear the weight of a heavy skull, features like the hips and shoulders might have reverted to become more like those of a small-brained hominin.”120
With such a ‘cover all the bases’ collection of evolutionary just-so stories there can hardly be any complaint if a non-evolutionary scenario in interpreting Homo naledi is provided here.
As argued in the preceding sections of this article, one reason why Homo naledi lacks several so-called “derived traits of the shoulder, trunk, and hip shared by H. erectus and H. sapiens”119 may be because there were individuals in the Dinaledi and Lesedi Chambers of the Rising Star cave system who had suffered from development pathology, most likely cretinism. Cretinism brought about by environmental iodine deficiency (cretins being the offspring of mothers with severe iodine deficiency) is not a genetic disorder,121 and can occur anywhere in the world where there is iodine deficiency in the food chain. As such it can affect entire populations in an environment where iodine deficiency is endemic. “Endemic cretinism occurs throughout the world, but its morphological traits vary substantially”.122 If individuals assigned to Homo naledi are cretins, then it makes more sense that they come from robust human populations, such as Homo erectus, because of their greater similarity in skeletal features to the latter than to modern humans. One explanation why robust humans (such as Homo erectus, Homo heidelbergensis and Neandertals) were more robust (heavily built) and/or different in morphology to modern humans is that this could reflect differences in development of these pre-Flood and early post-Flood humans, linked to longevity.123
According to evolutionist Charles Oxnard: “Of course, all cretins are not identical. The effects of the deficiency vary to greater or lesser degree. Their genetic heritages can also be expected to influence the picture.”124 Oxnard makes the following revealing statement:
“It is remarkable that so many features similar to those normally present in great apes, in Australopithecus and Paranthropus, and in early Homo (e.g., H. erectus and even to some degree, H. neanderthalensis) but not in modern H. sapiens are generated in humans by growth deficits due to the absence of thyroid hormone. In other words, many of the pathological features of cretinism mimic the primitive characters of evolution making it easy to mistake pathological features for primitive characters.”125
If a modern human with cretinism can have many pathological features that mimic the so-called ‘primitive’ features of evolution, it is highly likely that robust humans, such as Homo erectus, with cretinism will have as many, if not even more such features, yielding individuals that look like members of Homo naledi.
Almost as intriguing concerning the identity of the strange Homo naledi fossils is how the remains ended up in the inaccessible Dinaledi Chamber. I addressed this issue somewhat in an earlier paper, regarding this chamber.9 A deliberate body disposal scenario is considered the most plausible explanation by the authors.126 Currently there is only evidence of there ever having been one entrance to the Dinaledi Chamber. With the discovery of more Homo naledi fossils in another chamber (Lesedi Chamber) of the Rising Star cave system which, although having four access routes from the surface,127 is still difficult to get into, but not as difficult as the Dinaledi Chamber, the mystery of how the skeletal remains ended up in either chamber deepens. Berger et al. propose “that funerary caching by H. naledi is a reasonable explanation for the presence of remains in the Dinaledi and Lesedi Chambers”.128 However, the truth of how the fossils got there is still very much a mystery.
References and notes
- Dirks, P.H.G.M. et al., The age of Homo naledi and associated sediments in the Rising Star Cave, South Africa, eLife 6:e24231, 2017 | doi:10.7554/eLife.24231. Return to text.
- Hawks, J. et al., New fossil remains of Homo naledi from the Lesedi Chamber, South Africa, eLife 6:e24232, 2017 | doi:10.7554/eLife.24232. Return to text.
- Berger, L.R. et al., Homo naledi and Pleistocene hominin evolution in subequatorial Africa, eLife 6:e24234, 2017 | doi:10.7554/eLife.24234. Return to text.
- Berger, L.R. et al., Homo naledi, a new species of the genus Homo from the Dinaledi Chamber, South Africa, eLife 4:e09560, 2015 | doi:10.7554/eLife.09560. Return to text.
- Dirks, P.H.G.M. et al., Geological and taphonomic context for the new hominin species Homo naledi from the Dinaledi Chamber, South Africa, eLife 4:e09561, 2015 | doi:10.7554/eLife.09561. Return to text.
- Hawks, ref. 2, See comments section at end of paper, using link: elifesciences.org/content/6/e24232. Return to text.
- Hawks, J., More secrets of human ancestry emerge from South African caves, theconversation.com, 9 May 2017. Return to text.
- Dirks, ref. 1, pp. 1, 34. Return to text.
- Line, P., The puzzling Homo naledi: a case of variation or pathology in Homo erectus?, 19 November 2015, creation.com/puzzling-homo-naledi. Return to text.
- Line, P., The mysterious Rising Star fossils, J. Creation 30(3):88–96, 2016. Return to text.
- Berger, ref. 4, p. 22. Return to text.
- Williams, S.A. et al., The vertebrae and ribs of Homo naledi, J. Hum. Evo. 104:136–154, 2017 | doi: 10.1016/j.jhevol.2016.11.003. Return to text.
- Williams, ref. 12, p. 152. Return to text.
- Hawks, ref. 2, pp. 27–28. Return to text.
- Hawks, ref. 2, p. 29. Return to text.
- Berger, ref. 4, pp. 18, 22. Return to text.
- Williams, ref. 12, p. 150. Return to text.
- Walker, A. and Shipman, P., The Wisdom of Bone, Phoenix, London, pp. 196–197, 1997. Return to text.
- Tattersall, I., The Strange Case of The Rickety Cossack, Palgrave Macmillan, New York, NY, pp. 203, 2015. Return to text.
- Tattersall, ref. 19, pp. 203–204. Return to text.
- Ortner, D.J. and Hotz, G., Skeletal manifestations of hypothyroidism from Switzerland, Am. J. Phys. Anthropol 127:5, 2005 | 10.1002/ajpa.20033. Return to text.
- Ortner, ref. 21, p. 3. Return to text.
- Larson, S.G., et al., Homo floresiensis and the evolution of the hominin shoulder, J. Hum. Evol. 53(6):720, 2007 | doi: 10.1016/j.jhevol.2007.06.003. Return to text.
- Feuerriegel, E.M. et al., The upper limb of Homo naledi, J. Hum. Evol. 104:161–162, 169, 2017 | doi: 10.1016/j.jhevol.2016.09.013. Return to text.
- Feuerriegel, ref. 24, pp. 162, 169. Return to text.
- Hawks, ref. 2, p. 23. Return to text.
- Larson, S.G., Evolutionary transformation of the hominin shoulder, Evol. Anthr. 16:178, 2007 | doi: 10.1002/evan.20149. Return to text.
- Lordkipanidze, D. et al., Postcranial evidence from early Homo from Dmanisi, Georgia, Nature 449(7160):306–307, 2007 | doi:10.1038/nature06134. Return to text.
- Eckhardt, R.B. et al., Rare events in earth history include the LB1 human skeleton from Flores, Indonesia, as a developmental singularity, not a unique taxon, PNAS 111(33):11961–11966, 2014, Supporting Information Online, p. 6. Return to text.
- Morwood, M.J. et a l., Further evidence for small-bodied hominins from the Late Pleistocene of Flores, Indonesia, Nature 437(7061): 1016, 2005 | doi:10.1038/nature04022. Return to text.
- Larson, ref. 27, p. 177. Return to text.
- Roach, N.T. and Richmond, B.G., Clavicle length, throwing performance and the reconstruction of the Homo erectus shoulder, J. Hum. Evol. 80:112, 2015 | doi: 10.1016/j.jhevol.2014.09.004. Return to text.
- Feuerriegel, ref. 24, p. 169. Return to text.
- Hawks, ref. 2, pp. 21–22. Return to text.
- Aiello, L. and Dean, C., An Introduction To Human Evolutionary Anatomy, Academic Press, San Diego, pp. 353–355, 1990. Return to text.
- Cartmill, M. and Smith, F.H., The Human Lineage, Wiley-Blackwell, New Jersey, p. 174, 2009. Return to text.
- Larson, S.G., Evolution of the hominin shoulder: Early Homo, In: F.E. Grine, J.F. Fleagle and R.E. Leakey (Editors), The First Humans – Origin and Early Evolution of the Genus Homo, Springer, p. 68, 2009. Return to text.
- Hawks, ref. 2, p. 6. Return to text.
- Feuerriegel, ref. 24, p. 155. Return to text.
- Feuerriegel, ref. 24, pp. 161, 166–167. Return to text.
- Hawks, ref. 2, pp. 23–24. Return to text.
- Kivell, T.L. et al., The hand of Homo naledi, Nat. Commun. 6:8431, p. 2, 2015 | doi: 10.1038/ncomms9431. Return to text.
- Kivell, ref. 42, pp. 5–7. Return to text.
- Kivell, ref. 42, p. 6. Return to text.
- Shreeve, J., Mystery man, National Geographic 228:52, October 2015. Return to text.
- Kivell, T.L., Evidence in hand: recent discoveries and the early evolution of human manual manipulation, Phil. Trans. R. Soc. B 370(682):20150105, p. 6, 2015 | dx.doi.org/10.1098/rstb.2015.0105. Return to text.
- Richmond, B.G., Biomechanics of phalangeal curvature, J. Hum. Evol. 53(6):689, 2007 | doi:10.1016/j.jhevol.2007.05.011. Return to text.
- Larson, S.G., et al., Descriptions of the upper limb skeleton of Homo floresiensis, J. Hum. Evol. 57(5):567, 2009 | doi: 10.1016/j.jhevol.2008.06.007. Return to text.
- Larson, ref. 48, p. 562. Return to text.
- Larson, ref. 48, pp. 562, 564, 567. Return to text.
- Oxnard, C. Obendorf, P.J. and Kefford, B.J., Post-cranial skeletons of hypothyroid cretins show a similar anatomical mosaic as Homo floresiensis, PLoS ONE 5(9): e13018, p. 1, 2010 | doi:10.1371/journal.pone.0013018. Return to text.
- Hawks, ref. 2, p. 30. Return to text.
- Berger, ref. 4, p. 17. Return to text.
- Wood, B. (Editor), Wiley-Blackwell Encyclopedia of Human Evolution, Volume I, John Wiley & Sons Ltd, West Sussex, UK, p. 403, 2011. Return to text.
- Gruss, L.T., Scmitt, D., The evolution of the human pelvis: changing adaptations to bipedalism, obstetrics and thermoregulation, Phil. Trans. R. Soc. B 370:20140063, p. 7, 2015 | dx.doi.org/10.1098/rstb.2014.0063. Return to text.
- Langdon, J.H., The Human Strategy, Oxford University Press, New York, pp. 117–118, 2005. Return to text.
- VanSickle, C., et al., Primitive pelvic features in a new species of Homo, p. 13; notes from talk presented at the 16 April 2016 session on Homo naledi at the 2016 meeting of the American Association of Physical Anthropologists in Atlanta, GA. carolinevansickle.com , accessed 21 April 2016. Return to text.
- Hawks, J., Is Homo naledi just a primitive version of Homo erectus?, johnhawks.net, 19 September 2015. Return to text.
- Oxnard, C., Ghostly Muscles, Wrinkled Brains, Heresies and Hobbits, World Scientific, Singapore, p. 319, 2008. See also Oxnard, ref. 51, p. 3. Return to text.
- Jungers, W.L. et al., Descriptions of the lower limb skeleton of Homo floresiensis, J. Hum. Evol. 57(5):538, 2009 | https://doi.org/10.1016/j.jhevol.2008.08.014. Return to text.
- Shreeve, Ref. 45, p. 57. Return to text.
- Harcourt-Smith, W.E.H. et al., The foot of Homo naledi, Nat. Commun. 6:8432, 2015 | doi: 10.1038/ncomms9432. Return to text.
- Gibbons, A., New human species discovered, Science 349(6253):1150, 2015 | doi: 10.1126/science.349.6253.1149. Return to text.
- DeSilva, J.M. and Throckmorton, Z.J., Lucy’s flat feet: The relationship between the ankle and rearfoot arching in early hominins, PLoS ONE 5(12): e14432, p. 1, 2010 | doi:10.1371/journal.pone.0014432. Return to text.
- Wits University Homo naledi Fact Sheet: Questions and Answers, p. 9, wits.ac.za, 10 September 2015. Return to text.
- Harcourt-Smith, ref. 62, p. 4. Return to text.
- Harris, E.J. et al., Diagnosis and treatment of pediatric flatfoot, J Foot Ankle Surg. 43(6):341, 2004 | doi: 10.1053/j.jfas.2004.09.013. Return to text.
- Lee, M.S. et al., Diagnosis and treatment of adult flatfoot, J Foot Ankle Surg. 44(2):78, 2005 | doi: 10.1053/j.jfas.2004.12.001. Return to text.
- Harcourt-Smith, ref. 62, p. 6. Return to text.
- Berger, ref. 4, p. 18. Return to text.
- Berger, ref. 4, p. 23. Return to text.
- Berger, ref. 4, p. 21. Return to text.
- Berger, ref. 4, pp. 8, 21. Return to text.
- Wood, ref. 54, p. 398. Return to text.
- Ruff, C.B. and Higgins, R., Femoral neck structure and function in early hominins, Am J Phys Anthropol. 150(4):517, 2013. | doi: 10.1002/ajpa.22214. Return to text.
- Marchi, D. et al., The thigh and leg of Homo naledi, J. Hum. Evol. 104:180, 2017 | 10.1016/j.jhevol.2016.09.005 Return to text.
- Marchi, ref. 76, pp. 174, 202. Return to text.
- Hawks, ref. 2, p. 33. Return to text.
- Hawks, ref. 2, pp. 30–32, 2017. Return to text.
- Hawks, ref. 2, p. 34. Return to text.
- Papageorgopoulou, C., Staub, K. and Ruhl, F., Hypothyroidism in Switzerland, In: M. Harleck, K. von Heyking and H. Schwarzberg (Editors), Sickness, Hunger, War, and Religion: Multidisciplinary Perspectives, Rachel Carson Center Perspectives, pp. 78, 84, 2012/13. Return to text.
- Ortner, ref. 21, p. 1, Return to text.
- Stringer, C., The Many Mysteries of Homo naledi, eLife 4:e10627, p. 2, 2015 | doi:10.7554/eLife.10627. Return to text.
- Berger, ref. 4, p. 20. Return to text.
- Berger, ref. 4, pp. 19–20. Return to text.
- Lordkipanidze, D., Ponce de Leόn, M.S., Margvelashvili, A., Rak, Y., Rightmire, G.P., Vekua, A. and Zollikofer, P.E., A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo, Science 342(6156):326–331, 2013 | doi: 10.1016/j.jhevol.2016.09.005. Return to text.
- Line, P., New Dmanisi skull threatens to bring the house down, 29 October 2013; creation.com/dmanisi. Return to text.
- Hartley, R., Some bones to pick, timeslive.co.za ,18 September 2015. Return to text.
- Laird, M.F. et al., The skull of Homo naledi, J. Hum. Evol. 104:121, 2017 | doi: 10.1016/j.jhevol.2016.09.009. Return to text.
- Schroeder, L. et al., Skull diversity in the Homo lineage and the relative position of Homo naledi, J. Hum. Evol. 104:131, 2017 | doi: 10.1016/j.jhevol.2016.09.014. Return to text.
- Rightmire, G.P. et al., Skull 5 from Dmanisi: Descriptive anatomy, comparative studies, and evolutionary significance, J. Hum. Evol. 104:72, 2017 | doi: 10.1016/j.jhevol.2017.01.005. Return to text.
- Hawks, ref. 2, p. 17. Return to text.
- Hawks, ref. 2, p. 11. Return to text.
- Gibbons, A., Newest member of human family is surprisingly young, Science 356(6338):571, 2017 | doi: 10.1126/science.356.6338.571. Return to text.
- Brown, P., LB1 and LB6 Homo floresiensis are not modern human (Homo sapiens) cretins, J. Hum. Evol. 104:203, 2012 | https://doi.org/10.1016/j.jhevol.2011.10.011. Return to text.
- Schwartz, J.H., Tattersall, I. and Chi, Z., Comment on “A Complete Skull from Dmanisi, Georgia, and the Evolutionary Biology of Early Homo”, Science 344(6182):360-a, 2014 | doi: 10.1126/science.1250056. Return to text.
- Note that brain size correlates with the cranial capacity, i.e. the volume of the cranial cavity (endocranial volume), measured in cubic centimeters (cc), although in actuality it is slightly less, as not all of the cavity is occupied by the brain. Return to text.
- Berger, ref. 4, p. 2. Return to text.
- Berger, ref. 4, p. 30. Return to text.
- Hawks, ref. 2, p. 14. Return to text.
- Spoor, F., Implications of new early Homo fossils from Ileret, east of Lake Turkana, Kenya, Nature 448(7154): 688, 2007 | doi:10.1038/nature05986. Return to text.
- Rightmire, ref. 91, p. 55. Return to text.
- Kubo, D., Kono, R.T. and Kaifu, Y., Brain size of Homo floresiensis and its evolutionary implications, Proceedings of the Royal Society B, 280: 20130338, p. 1, 2013 | dx.doi.org/10.1098/rspb.2013.0338. Return to text.
- Molnar, S., Races, Types, and Ethnic Groups, Prentice-Hall Inc., NJ, pp. 56–57, 1975. Return to text.
- Skoyles, J.R., Human evolution expended brains to increase expertise capacity, not IQ, Psycoloquy 10(002), 1999; cogsci.ecs.soton.ac.uk. Return to text.
- Using a formula linking brain volume to cranial capacity, the cranial capacity of Lyon’s cranium can be estimated to be about 660 cc. See: Aiello, L. and Dunbar, R., Neocortex size, group size and the evolution of language, Curr. Anthropol. 34(2):185, 1993. The formula derived by the authors is: Log10(B) = 3.015 + 0.986 Log10(C), where B is the total brain size (brain volume) in mm3 and C is the internal cranial capacity in cubic centimeters (cc). Return to text.
- Hawks, ref. 2, p. 45. Return to text.
- Obendorf, P.J., Oxnard, C.E. and Kefford, B.J., Are the small human-like fossils found on Flores human endemic cretins?, Proc Biol Sci. 275(1640):1290, 2008 | doi: 10.1098/rspb.2007.1488. Return to text.
- Hawks, J. and Berger, L.R., The impact of a date for understanding the importance of Homo naledi, Trans. R. Soc. S. Afr. 71(2):127, 2016 | doi: 10.1080/0035919X.2016.1178186. Return to text.
- Dembo, M., et al., The evolutionary relationships and age of Homo naledi: An assessment using dated Bayesian phylogenetic methods, J. Hum. Evol. 97:17, 2016 | doi: 10.1016/j.jhevol.2016.04.008. Return to text.
- Wong, K., Mystery human, SciAm 314(3):29, March 2016 | doi:10.1038/scientificamerican0316-28. Return to text.
- Sarfati, J., Refuting Compromise, Second Edition, Creation Book Publishers, Atlanta, Georgia, pp. 372–383, 2011. Return to text.
- Snelling, A.A, Earth’s Catastrophic Past: Geology, Creation & The Flood, Volume 2, Institute for Creation Research, Dallas, Texas, pp. 797–864, 2009. Return to text.
- Bower, B., ‘Java Man’ takes age to extremes, sciencenews.org, 16 April 2010. Return to text.
- Dirks, ref. 1, p. 26. Return to text.
- Dirks, ref. 1, p. 34. Return to text.
- Berger, ref. 3, p. 2. Return to text.
- Berger, ref. 3, p. 13. Return to text.
- Berger, ref. 3, p. 7. Return to text.
- Barras, C., Meet Neo, the most complete Homo naledi, New Scientist 234(3125):9, 13 May 2017. Return to text.
- Oxnard, ref. 59, pp. 303, 342. Return to text.
- Dobson, J.E., The iodine factor in health and evolution, Geogr Rev 88(1):5, 1998 | doi: 10.2307/215869. Return to text.
- Line, P., Explaining robust humans, J. Creation 27(3):64–71, 2013. Return to text.
- Oxnard, ref. 59, p. 320. Return to text.
- Oxnard, ref. 59, p. 342. Return to text.
- Dirks, ref. 5, p. 30. Return to text.
- Hawks, ref. 2, p. 3. Return to text.
- Berger, ref. 3, p. 12. Return to text.








Readers’ comments
Comments are automatically closed 14 days after publication.