Journal of Creation 25(3):42–45, December 2011
Browse our latest digital issue Subscribe
‘Transitional form’ in mammal ear evolution—more cacophony
A new fossil mammal, Liaoconodon hui, was found in Liaoning and ‘dated’ at 120 Ma (million years) old. It has been hailed as a transitional form between reptiles and modern mammals because of its unique ear bone morphology. However, it is ‘dated’ 40–75 Ma after the appearance of the first fully formed mammalian middle ear. The middle ear morphology, though different from extant mammals in their adult form, still doesn’t provide evidence of the crucial changes required to go from the reptilian to the mammalian middle ear. Liaoconodon is clearly a mammal, though it possesses either an embryonic mammalian middle ear structure or a new functional morphology. Either interpretation is consistent with the biblical model, but produces numerous homoplasies in the evolutionary model. Therefore the Bible provides a better explanation of the ear morphology of Liaoconodon than evolution.
A new fossil has been found in Liaoning and has been dubbed Liaoconodon hui and ‘dated’ to the Early Cretaceous, at 120 Ma (million years) old.1 It has been hailed as a transitional form between reptiles and modern mammals because of its unique ear bone morphology. It has been paraded on many of the science news websites as the long-sought fossil mammal with a transitional middle ear.2,3,4 This is rather interesting, considering that the reptile-to-mammal ‘lineage’ is often held up as the most complete and irrefutable fossil evidence for microbes-to-man evolution. However, the reptile-to-mammal ‘transition’ as a whole has many problems,5 and as we shall see, Liaoconodon doesn’t help the picture either.
What of the fossil context?
This fossil is from the Chinese Jehol Group, where it seems truckloads of ‘missing links’ (mostly of the dino-to-bird kind, but others such as the ‘early’ mammal Yanoconodon have been discovered) have been hiding until the last 15–20 years.6 And since there is one well-known fraud to have come from there (the Archaeoraptor hoax),7 one wonders if that is the only fossil fraud to have been perpetrated on the scientific establishment. I’m not suggesting that this or any particular find from the Jehol group is fraudulent, but merely pointing out that there is reason for a priori scepticism about fossils from there.
There also seems to be a consistent factor concerning fossils from this part of the world: they all seem to be dated many millions of years too late to shed any real light on evolution. Liaoconodon, which has what the authors describe as a “transitional mammalian middle ear” (TMME), is ‘dated’ to 120 Ma, which is 75 Ma younger than the first “definitive mammalian middle ear” (DMME), Hadrocodium.8 If this were a ‘mere’ 5 Ma difference, then it could be much easier to conclude that the ‘ghost lineage’ created by this paradox is just bad luck. However, when the age difference is greater than a geologic period, then it just becomes a brazenly ad hoc ‘solution’ to preserve evolutionary cladistics.9
An ‘earie’ morphology
This ear arrangement found in Liaoconodon and other ‘early’ mammals is not completely absent in modern mammals. The key morphological feature discussed by Meng et al., Meckel’s cartilage, is part of the developmental process of the mammalian ear and jaw, and the jaw of other tetrapods. The difference between living mammals and Liaoconodon is that while the Meckel’s cartilage dissolves during embryonic development of living mammals, it is present in Liaoconodon (allegedly retained during evolution, eventually becoming bone).1 Therefore, this could be akin to gills in the adult axolotl:10 a juvenile structure that has been preserved in an adult. This is an interpretation that was favoured in the closely related Yanoconodon:
“Paedomorphosis, or retention of fetal or juvenile characteristics of ancestors and relatives through developmental heterochrony [differences in developmental timing], is a common phenomenon in vertebrate evolution. The heterochronic (‘premature’) ossification of Meckel’s cartilage in eutriconodonts is the immediate cause for this paedomorphic connection of middle ear and mandible, and is why there is an overall homoplastic distribution among therians (with DMME), eutriconodonts (without DMME), monotremes (with DMME) and pre-mammalian relatives (without DMME).”11
Another possible interpretation is that Liaoconodon and other ‘early’ mammals have a completely functional auditory system morphologically and functionally distinct from living mammals. Meng et al. favour this interpretation:
“The transference from the mandibular middle ear (MME) to the TMME and then to the DMME represents two distinct evolutionary stages, each involving several morphological changes.”12
This would be explained by slight differences in the same basic developmental plan for the mammalian ear. Neither interpretation demands evolution, because the first involves information loss, and the second is simply common design. Both interpretations can be incorporated into a biblical picture of life’s diversity, as long as one abandons the universal common descent assumption of evolution.
Haeckel returns!
However, Meng et al. explain the reason for the morphological differences in a rather unexpected way:
“Instead of being a paedomorphic resemblance, an alternative hypothesis is that the persistent Meckel’s cartilage in Mesozoic mammals, along with features such as lack of the manubrium and a partial ectotympanic, represents a phylogenetic stage in mammalian evolution, and that the embryonic pattern of modern mammals recapitulates the phylogenetic changes [emphasis added].”12
Haeckel returns! But embryonic recapitulation is about as far removed from reality in this instance as Haeckel’s formulation was in the 19th century. For a start, Liaoconodon would have to be an example of embryonic recapitulation running in reverse. There is solid evidence that other ‘early’ mammals, multituberculates and Hadrocodium, already possessed the DMME condition 40 Ma and 75 Ma before Liaoconodon lived, respectively, according the evolutionary scheme.
Moreover, embryonic recapitulation is only an explanation of pattern, not an explanation of process. It doesn’t tell us how the developmental process was re-patterned to give completely different functional morphologies. Just because stages of alleged evolution appear in a developmental sequence, it does not mean those stages are all functional during ontogeny (either for hearing or as a jaw joint). This is the key difference between ontogeny and phylogeny—only the final product of ontogeny has to be properly functional, but every generation of phylogeny must be functional for evolution to have any plausibility. Neither does it tell us anything about how the crucial morphological changes necessary to move from the typical reptilian (stapes-only) middle ear to the mammalian (malleus-incus-stapes) middle ear (whether ‘transitional’ or ‘definitive’) actually happened.

The futility of fossils
Fossil evidence usually has a fundamental weakness: we can never see the bones, even if found in their correct articulation, in their full physiological context. Functional or phylogenetic inferences based solely on the intricacies of bone structure in extinct fossils have gotten palaeontologists into many problems before, as Lieberman points out:
“Bones have generally low degrees of heritability because they form parts of complex, integrated functional units that are subject not only to many genes with multiple effects (pleiotropism), but also to a large number of nongenetic influences. It is therefore difficult to divide bones into discrete, independent units of phylogenetic information. For these reasons, bones and other aspects of morphology can yield reasonably correct results for phylogenetic analyses of high-level taxonomic units, but become increasingly less reliable at lower taxonomic levels, such as species.”13
Lieberman said this in the context of human evolution, where the features for comparison are often quite large, over 10 cm long. However, his comments only become more pertinent when we’re dealing with the three smallest bones in the mammalian body in mammals that are often only 2–3 cm long in toto. Evolutionists need to be extremely cautious about any phylogenetic inferences they make.
Finally, for evolution to have any plausibility based on the fossils, the ‘processes’ invoked to explain the patterns need a firm empirical basis. However, evolution suffers from numerous problems. There is widespread testimonial evidence for the biblical Flood in the universal spread of Flood legends, of which the Genesis account is the most realistic and reliable.14 This provides a solid conceptual basis for understanding fossil distribution, which evolution lacks.15 Microbes-to-man evolution suffers from the lack of a viable empirical mechanism. The empirical evidence testifies to universal and inevitable degradation of biological information—the opposite of what evolution requires.16 The basic ‘a watch implies a watchmaker’ analogy with respect to biology is valid, despite attempts to refute it.17,18 Finally, fossil evidence, because it is so fragmentary and sparse, is weak and open to contradictory interpretations.19
‘Transitional forms’ leave more transitions to explain
Meng et al. also provide a rather detailed argument for the functionality of the TMME in Liaoconodon.20 Let’s assume that their description of the functioning of the TMME is accurate. We then have two large morphological gaps in the place of one huge one. If the structural evolution went MME–TMME–DMME, then we have not one, but two significant re-patternings of the ear that have to be explained. The morphological disparity between the morganucodonts and Liaoconodon remains huge, and they’ve now added another large gap in morphology between some ‘early’ mammals and extant mammals! Note that we are talking about two large morphological gaps—the sizes of the gaps matter, as Woodmorappe explains:
“In particular, as long as such things as half-legs/half-wings, or three-quarter scales/one-quarter feathers, are not found as fossils, the discontinuities among such things as reptiles and birds remain large. This remains the case whether or not some ‘transitional’ fossil can be thought of as replacing one larger gap into two smaller but nevertheless still large gaps.”21
The crucial morphological changes that are required to evolve a mammalian middle ear (whether a DMME or a TMME) from a jaw joint are still conspicuously absent from the fossil record.22 Liaoconodon possesses a malleus and incus, like all other mammals—they don’t form part of a jaw joint. The closest ‘relatives’ of mammals, the morganucodonts, had a double-jointed jaw—one mammal-like jaw joint and one slender reptile-like jaw joint. It has been speculated that the reptilian jaw joint in moganucodonts served a supporting function in supporting its middle ear.23 However, the fact that the morphology is vastly different from the typical mammalian middle ear articulation,24 and that it remains attached to the dentary (mandible), still points to the vast difference in middle ear morphology that morganucodonts have from all mammals. So, the reptile-like jaw joint is still primarily a jaw joint, and it has a completely different ear structure, the mandibular middle ear (MME) (figure 1).
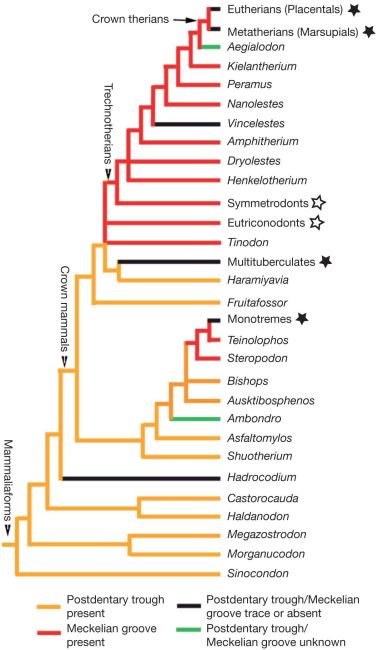
What’s more, the second gap (TMME to DMME) has to be breached at least five times independently according to Meng et al.’s cladogram—once each for Hadrocodium, monotremes, Vincelestes, therians (placentals and marsupials) and multituberculates (figure 2). And while this gap is not as large as the one between morganuconodonts and Liaoconodon, it is still a significant change in functional morphology.25 Meng et al. are completely aware of this, but their faith in the power of evolution is unshakeable: “The ear morphology of Liaoconodon represents a transitional stage in the evolution of mammalian middle ears regardless of how many times the DMME evolved.”26
This ‘transitional form’ was obviously successful because, from an evolutionary perspective, it had to have lasted at least 75 Ma, and should have first arisen before Hadroconium, which is typically ‘dated’ around 195 Ma old. That raises a problem: why did the DMME evolve so often from an obviously successful articulation, especially given that Meng et al. tell us that “the TMME must be more efficient in airborne sound hearing than the mandibular middle ear”?26 That “the middle ear of Liaoconodon is not so efficient as in extant mammals”26 is no excuse because evolution knows no direction or purpose.
Meng et al., like most evolutionists, also assume that evolution is the only explanation for the developmental process of the mammalian ear. However, it also makes sense that a single designer would modify the same developmental program to create different creatures, otherwise it might look like life was the product of more than one designer.27 Since Liaoconodon can be explained according to biblical creation using Meng et al.’s own interpretation of the functional morphology, it’s disingenuous to portray evolution as having all the answers.
Conclusions
Liaoconodon seems to have a distinct middle ear bone articulation, though it is three-bone (malleus-incus-stapes), and thus still distinctly mammalian. It could be a paedomorphic trait, and as such is a loss in information from the DMME condition. However, it could also be a completely new functional morphology, though still distinctly mammalian. Evolution can only be seen in this ‘transitional form’ if one presupposes evolution in the first place. The crucial transformation required to decouple the extra middle ear bones in mammals from the reptilian jaw joint is still not evidenced in the fossils. This study also fails to appreciate why ontogeny is not a good guide for understanding phylogeny. Just because an embryo goes through a stage that looks like the adult condition of a presumed ‘ancestral’ trait, it does not mean that the embryonic trait was ever, in any way, functional in the genealogy of the organism with the ‘derived’ trait. And the fossil is dated far too late in the evolutionary scheme to work as a chronological intermediate. Therefore, there is no reason to postulate evolution to explain this curious fossil. Rather, it makes better sense to envisage a single designer modifying the same basic developmental plan for his individual creatures, as the Bible declares.
References
- Meng, J., Wang, Y. and Li, C., Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont, Nature 472:181–185, 2011. Return to text.
- Long-sought fossil mammal with transitional middle ear found, PhysOrg com, 13 April 2011; www.physorg.com. Return to text.
- O’Luanaigh, C., Hear my chewin’: how jawbones evolved into ear bones, New Scientist, 13 April 2011; www.newscientist.com. Return to text.
- Welsh, J., Jaw-Dropping Find: Ancient Mammal’s Ear Bones, Livescience. com, 13 April 2011; www.livescience.com. Return to text.
- Woodmorappe, J., Mammal-like reptiles: major trait reversals and discontinuities, J. Creation (formerly TJ) 15(1):44–52, 2001. Return to text.
- Sibley, A., Chinese fossil layers and the uniformitarian re-dating of the Jehol Group, J. Creation 21(1):123–127, 2007. Return to text.
- Sarfati, J., Archaeoraptor—Phony ‘feathered’ fossil, 3 February 2000. Return to text.
- Luo, Z.-X., Crompton, A.W. and Sun, A.-L., A new mammaliaform from the Early Jurassic and evolution of mammalian characteristics, Science 292(5521):1535–1540, 2001. Return to text.
- Doyle, S., Plucking the dinobird, 28 September 2007. Return to text.
- Dykes, J., The Axolotl: the fish that walks? Creation 27(4):21–23, 2005; creation.com/the-axolotl-the-fish-that-walks. Return to text.
- Luo, Z.-X., Chen, P.-J., Li, G. and Chen, M., A new eutriconodont mammal and evolutionary development in early mammals, Nature 446:288–293, 2007. Return to text.
- Meng et al., ref. 1, p. 183. Return to text.
- Lieberman, D.E., Homology and hominid phylogeny: problems and potential solutions, Evolutionary Anthropology 7:142–151, 1999. Return to text.
- Sarfati, J., Noah’s Flood and the Gilgamesh Epic, Creation 28(4):12–17, 2006;. See also: Osanai, N., A comparative study of the flood accounts in the Gilgamesh Epic and Genesis, MA Thesis, Wesley Biblical Seminary, USA, 2004. Return to text.
- Reed, J.K., Cuvier’s analogy and its consequences: forensics vs testimony as historical evidence, J. Creation 22(3):115–120, 2008. Return to text.
- Williams, A., Mutations: evolution’s engine becomes evolution’s end! J. Creation 22(2):60–66, 2008. Return to text.
- Weinberger, L., Grand undertaking: a review of God’s Undertaker: Has Science Buried God? by John C. Lennox, J. Creation 23(3):35–38, 2009. Return to text.
- Sarfati, J., By Design, Creation Ministries International, Brisbane, Australia, 2008. Return to text.
- Doyle, S., ‘Oldest’ fossil shrimp? J. Creation 25(1):3–4, 2011. Return to text.
- Meng et al., ref. 1, pp. 182–183. Return to text.
- Woodmorappe, J., Does a ‘transitional form’ replace one gap with two gaps? J. Creation (formerly CENTJ) 14(2):5–6, 2000. Return to text.
- Camp, A., Reappraising the ‘Crown Jewel’, Creation Matters 3(5):1–5, 1998; www.trueorigin.org/therapsd.asp. Return to text.
- Meng et al., ref. 1, p. 181. Return to text.
- Meng et al., ref. 1, supplementary information, pp. 36–39. Return to text.
- Meng et al., ref. 1, supplementary information, pp. 39–40. Return to text.
- Meng et al., ref. 1, p. 184. Return to text.
- Batten, D., Review of The Biotic Message: Evolution versus Message Theory, J. Creation 11(3):292–298, 1997. Return to text.




Readers’ comments
Comments are automatically closed 14 days after publication.