Origin of life
An explanation of what is needed for abiogenesis (or biopoiesis)
Last updated February 2024.
Introduction
The origin of life is also known as abiogenesis or sometimes chemical evolution.
Life is based on long information-rich molecules such as DNA and RNA that contain instructions for making proteins, upon which life depends. But the reading of the DNA/RNA to make proteins, and the replication of DNA or RNA to make new cells (reproduction, the mark of ‘life’) both depend on a large suite of proteins that are coded on the DNA/RNA. Both the DNA/RNA and the proteins need to be present at the same time for life to begin—a serious chicken-and-egg conundrum.
Thus, the origin of life is a vexing problem for those who insist that life arose through purely natural processes (physics and chemistry alone).
Some evolutionists claim that the origin of life is not a part of evolution. However, probably every evolutionary biology textbook has a section on the origin of life in the chapters on evolution. The University of California, Berkeley, has the origin of life included in their ‘Evolution 101’ course, in a section titled “From Soup to Cells—the Origin of Life”.1 High-profile defenders of ‘all-things-evolutionary’, such as P.Z. Myers and Nick Matzke, agree that the origin of life is part of evolution, as does Richard Dawkins.2
A well-known evolutionist of the past, G.A. Kerkut, did make a distinction between the General Theory of Evolution (GTE), which included the origin of life, and the Special Theory of Evolution (STE) that only dealt with the diversification of life (the supposed topic of Darwin’s 1859 book).3
It is only recently that some defenders of evolution have tried to divorce the origin of life from consideration. It’s probably because the hope of finding an answer is rapidly fading, as one scientific discovery after another of sophisticated machinery in even the simplest living cells makes the problem of a naturalistic origin ever more difficult.
So, what do we need to get life? We can break the problem of the origin of life into a number of topics in an attempt to explain to non-scientists what is involved (although it still might be mind-stretching).

What is it that we have to obtain to produce a living cell? A living cell is capable of acquiring all the resources it needs from its surroundings and reproducing itself. The first cell had to be free-living; that is, it could not depend on other cells for its survival because other cells did not exist. Parasites cannot be a model for ‘first life’ because they need existing cells to survive. This also rules out viruses and the like as the precursors to life as they must have living cells that they can parasitize to reproduce themselves. Prions, misshaped proteins that cause disease, have nothing to do with the origin of life because they can only ‘replicate’ by causing proteins manufactured by a cell to become misshaped.
The first things needed are the right ingredients. It’s bit like baking a cake; you can’t make a banana cake if you have no bananas or flour.
Getting all the right ingredients
Right here there is a major problem for chemical soup approaches to the origin of life: all the components have to be present in the same location for a living cell to have any possibility of being assembled. But necessary components of life have carbonyl (>C=O) chemical groups that react destructively with amino acids and other amino (–NH2) compounds. Such carbonyl-containing molecules include sugars,4 which also form the backbone of DNA and RNA. Living cells have ways of keeping them apart and protecting them to prevent such cross-reactions, or can repair the damage when it occurs, but a chemical soup has no such facility.
Cells are incredibly complex arrangements of simpler chemicals. I am not going to cover every chemical that a first cell would need; it would take a book and some to cover it. I am just going to highlight some of the basic components that have to be present for any origin-of-life scenario.
a. Amino acids
Living things are loaded with proteins; linear strings of amino acids. Enzymes are special proteins that help chemical reactions to happen (catalysts). For example, the enzyme amylase is secreted in our saliva and causes starch molecules from rice, bread, potatoes, etc., to break up into smaller molecules, which can then be broken down to their constituent glucose molecules. We can’t absorb starch, but we are able to absorb glucose and use it to power our bodies.
Some reactions necessary for life go so slowly without enzymes that they would effectively never produce enough product to be useful, even given billions of years.5
Other proteins form muscles, bone, skin, hair and all manner of the structural parts of cells and bodies. Humans can produce well over 100,000 proteins (possibly millions; nobody really knows exactly how many), whereas a typical bacterium can produce one or two thousand different ones.

Proteins are made up of 20 different amino acids (some microbes have an extra one or two). Amino acids are not simple chemicals and they are not easy to make in the right way without enzymes (which are themselves composed of amino acids); see Figure 1.
The 1953 Miller–Urey experiment, which almost every biology textbook still presents, managed to make some amino acids without enzymes. It is often portrayed as explaining ‘the origin of life’, but that is either very ignorant or very deceitful.
Although tiny amounts of some of the right amino acids were made, the conditions set up for the experiment could never have occurred on Earth; for example, any oxygen in the ‘atmosphere’ in the flask would have prevented anything from forming, and the evidence points to Earth always having had oxygen. Furthermore, some of the wrong types of amino acids were produced, as well as other chemicals that would ‘cross-react’, preventing anything useful forming.
The amino acids required for functional proteins could never have been made by anything like this experiment in nature.6 When Stanley Miller repeated the experiment in 1983 with a slightly more realistic mixture of gases, he only got trace amounts of glycine, the simplest of the 20 amino acids needed.7
Update 2021: There seems to be renewed enthusiasm in some quarters for injecting new life into the Miller-Urey experiment: Ouellette, J., Scientists recreated classic origin-of-life experiment and made a new discovery, arstechnica.com, 29 October 2021. This commentary hypes a finding of scientists that silica leached from the borosilicate glass flasks used by Urey contributed a catalytic function to the apparatus. They also detected a wider range of organic compounds than Miller-Urey. However, these findings do not change the conclusion that the Miller-Urey experiment is irrelevant to the origin of the first cell, for the reasons stated herein.
The origin of the correct mix of amino acids remains an unsolved problem (and see another major problem under ‘handedness’ below).
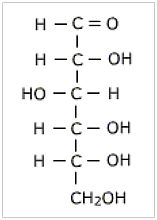
b. Sugars
Some sugars can be made just from chemistry without enzymes (which are only made by cells, remember). Sugars are supposed to have formed from naturally occurring formaldehyde in the presence of alkali by the formose (or Butlerov) reaction. However, the very same alkaline conditions that are needed for this reaction also destroy sugars such as ribose and glucose that are essential for life.
The formose reaction that is proposed for the formation of sugars also needs the absence of nitrogenous compounds, such as amino acids, because these react with the formaldehyde, and the sugars, to produce non-biological chemicals.
Ribose, the sugar that forms the backbone of RNA, and in modified form DNA, an essential part of all living cells, is especially problematic. It is an unstable sugar (it has a short half-life, or breaks down quickly) in the real world at near-neutral pH (neither acid nor alkaline).8
c. The components of DNA and RNA
How can we get the nucleotides that are the chemical ‘letters’ of DNA and RNA without the help of enzymes from a living cell? The chemical reactions require formaldehyde (H2C=O) to react with hydrogen cyanide (HC≡N). However, formaldehyde and cyanide (especially) are deadly poisons. They would destroy critically important proteins that might have formed!
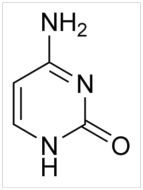
Cytosine (Figure 3), one of the five essential nucleotide bases of DNA and RNA, is very difficult to make in any realistic pre-biotic scenario and is also very unstable.8
DNA and RNA also have backbones of alternating sugars and phosphate groups. The problems with sugars are discussed above. Phosphates would be precipitated by the abundant calcium ions in sea water or cling strongly onto the surfaces of clay particles. Either scenario would prevent phosphate from being used to make DNA.
d. Lipids
Lipids (‘fats’) are essential for the formation of a cell membrane that contains the cell contents, as well as for other cell functions. The cell membrane, comprised of several different complex lipids, is an essential part of a free-living cell that can reproduce itself.
Lipids have much higher energy density than sugars or amino acids, so their formation in any chemical soup is a problem for origin-of-life scenarios (high energy compounds are thermodynamically much less likely to form than lower energy compounds).
The fatty acids that are the primary component of all cell membranes have been very difficult to produce, even assuming the absence of oxygen (a ‘reducing’ atmosphere). Even if such molecules were produced, ions such as magnesium and calcium, which are themselves necessary for life and have two charges per atom (++, i.e. divalent), would combine with the fatty acids, and precipitate them, making them unavailable.9 This process likewise hinders soap (essentially a fatty acid salt) from being useful for washing in hard water—the same precipitation reaction forms the ‘scum’.

Some popularisers of abiogenesis like to draw diagrams showing a simple hollow sphere of lipid (a ‘vesicle’) that can form under certain conditions in a test-tube. However, such a ‘membrane’ could never lead to a living cell because the cell needs to get things through the cell membrane, in both directions. Such transport into and out of the cell entails very complex protein-lipid complexes known as transport channels, which operate like electro-mechanical pumps. They are specific to the various chemicals that must pass into and out of the cell (a pump that is designed to move water will not necessarily be suitable for pumping oil). Many of these pumps use energy compounds such as ATP to actively drive the movement against the natural gradient. Even when movement is with the gradient, from high to low concentration, it is still facilitated by carrier proteins.
The cell membrane also enables a cell to maintain a stable pH, necessary for enzyme activity, and favourable concentrations of various minerals (such as not too much sodium). This requires transport channels (‘pumps’) that specifically move hydrogen ions (protons) under the control of the cell. These pumps are highly selective.10
Transport across membranes is so important that “20–30% of all genes in most genomes encode membrane proteins”.11 The smallest known genome of a free-living organism, that of the parasite Mycoplasma genitalium, codes for 26 transporters12 amongst its 482 protein-coding genes.
A pure lipid membrane would not allow even the passive movement of the positively-charged ions of mineral nutrients such as calcium, potassium, magnesium, iron, manganese, etc., or the negatively-charged ions such as phosphate, sulfate, etc., into the cell, and they are all essential for life. A pure-lipid membrane would repel such charged ions, which dissolve in water, not lipid. Indeed, a simple fat membrane would prevent the movement of water itself (try mixing a lipid like olive oil with water)!
Membrane transporters would appear to be essential for a viable living cell.
In the 1920s the idea that life began with soapy bubbles (fat globules) was popular (Oparin’s ‘coacervate’ hypothesis) but this pre-dated any knowledge of what life entailed in terms of DNA and protein synthesis, or what membranes have to do. The ideas were naïve in the extreme, but they still get an airing today in YouTube videos showing bubbles of lipid, even dividing, as if this were relevant to explaining the origin of life (see: Self-made cells? Of course not!).
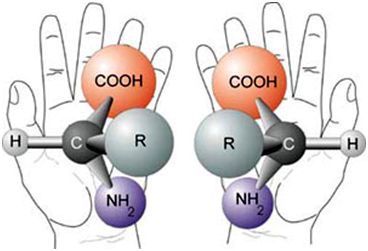
e. Handedness (chirality)
Amino acids, sugars, and many other biochemicals, being 3-dimensional, can usually be in two forms that are mirror images of one another; like your right and left hand are mirror images of each other. This is called handedness or chirality (Figure 5).
Now living things are based on biochemicals that are pure in terms of their chirality (homochiral): left-handed amino acids and right-handed sugars, for example. Here’s the rub: chemistry without enzymes (like the Miller–Urey experiment), when it does anything, produces mixtures of amino acids that are both right- and left-handed. It is likewise with the chemical synthesis of sugars (with the formose reaction, for example).13
Origin-of-life researchers have battled with this problem and all sorts of potential solutions have been suggested but the problem remains unsolved.14 Even getting 99% purity, which would require some totally artificial, unlikely mechanism for ‘nature’ to create, doesn’t cut it. Life needs 100% pure left-handed amino acids. The reason for this is that placing a right-handed amino acid in a protein in place of a left-handed one results in the protein having a different 3-dimensional shape. None can be tolerated to get the type of proteins needed for life.
What are the minimum requirements for a cell to live?
A minimal free-living cell that can manufacture its components using chemicals and energy obtained from its surrounding environment and reproduce itself must have:
A cell membrane. This separates the cell from the environment. It must be capable of maintaining a different chemical environment inside the cell compared to outside (as above). Without this, life’s chemical processes are not possible.
A way of storing the information or specifications that instructs a cell how to make another cell and how to operate moment by moment. The only known means of doing this is DNA and any proposals for it to be something else (such as RNA) have not been shown to be viable—and then there has still to be a way of changing from the other system to DNA, which is the basis of all known life.15
- A way of reading the information in (2) to make the cell’s components and also control the amount produced and the timing of production. The major components are proteins, which are strings (polymers) of hundreds to thousands of some 20 different amino acids. The only known (or even conceivable) way of making the cell’s proteins from the DNA specifications involves over 100 proteins and other complex co-factors. Involved are:
nano-machines such as RNA polymerase (smallest known type has ~4,500 amino acids),
gyrases, which twist/untwist the DNA spiral to enable it to be ‘read’ (again these are very large proteins),
ribosomes, sub-cellular ‘factories’ where proteins are manufactured, and
at least 20 transfer-RNA molecules; these select the right amino acid to be placed in the order specified on the DNA (all cells that we know of have at least 61 because most amino acids are specified by more than one DNA three-letter code). The transfer-RNAs have sophisticated mechanisms for making sure the right amino acid is selected according to the DNA code.
There are also mechanisms to make sure that the proteins made are folded three-dimensionally in the correct way that involve chaperones to protect the proteins from mis-folding, plus chaperonin folding ‘machines’ in which the proteins are helped to fold correctly. All cells have these.
A means of manufacturing the cell’s biochemical needs from the simpler chemicals in the environment. This includes a way of making ATP, the universal energy currency of life. All living cells today have ATP synthase, a phenomenally complex and efficient electric rotary motor to make ATP (or in reverse to create electric currents that drive other reactions and movement both inside and outside the cell).
A means of copying the information and passing it on to offspring (reproduction). A simulation of one cell division of the simplest known free-living bacterium (which ‘only’ has 525 genes) required 128 desktop computers working together for 10 hours.16
Whew! And that’s just the basics.
A greatly simplified animation of protein synthesis, which includes the action of RNA polymerase, ribosomes, transfer-RNAs, chaperonins, and chaperones. All living cells have this system of protein synthesis.
This gives some indication of what needs to happen for the first living cell to live.
An interesting project began some years ago to ascertain what could be the minimal cell that could operate in a free-living manner; that is, not dependent on another living organism. However, it did have available a nutrient-rich medium that provided a wealth of complex organic compounds such that the cell did not have to synthesize many of its needed biochemicals. This minimal cell is now known to need over 400 protein and RNA components,17 and of course that means that its DNA needs to be loaded up with the specifications for making these. That is, the DNA needs to have over 400 ‘genes’. We will come back to this later.
Polymer formation (polymerisation)
Life is not just composed of amino acids or sugars but it is loaded with polymers, which are strings, or chains, of simpler compounds joined together. A polysaccharide is a polymer of sugars. A protein is a polymer of amino acids and DNA and RNA are polymers of nucleotides. Polysaccharides are the simplest, where the links in the chain are normally the same sugar compound, such as glucose (making starch in plants or glycogen in animals). Proteins are much more complex, being chains of amino acids where each link in the chain can be one of 20 different amino acids. And there are four different links in DNA and RNA.
Now water is an essential ingredient of living cells; typical bacteria are about 75% water. Being the ‘universal solvent’, water is a necessary carrier for the various components of cells; it is the milieu in which it all happens.
Here is a huge problem for origin-of-life scenarios: when amino acids are joined together, for example, a water molecule is released. This means that in the presence of water, the reaction is pushed in the wrong direction, backwards; that is, proteins will fall apart, not build, unless the water is actively removed. A cell overcomes this by protecting the reaction site from water (inside ribosomes) and providing energy to drive this and the polymer formation. Thus, the formation of proteins of more than a few amino acids is a huge problem for all origin-of-life scenarios (and adding more time does not solve the problem; they just fall apart more).
Polymer formation also requires that the ingredients (monomers) that are joined together are bi-functional. That simply means that the amino acids for making proteins (or sugars for making polysaccharides) have at least two active sites that will allow another amino acid (or sugar) to be joined to each end. A protein-forming amino acid will have at least one amino group (-NH2) and one carboxyl group (-COOH), with the amino group of one amino acid joining to the carboxyl group of another, thus growing the chain. A compound with only one active site (mono-functional) would terminate the formation of the chain. The problem for origin-of-life scenarios is that any proposed chemical reactions that produce some amino acids also produce mono-functional ones that terminate protein formation.18
However, some important amino acids that are critically important in making proteins are tri-functional; they have an extra carboxyl group or an extra amine group, not just one. Aspartic acid and lysine are examples. This means that they cannot join in the correct manner without enzymes to help them; many of their bonds will be wrong. This would appear to be an insurmountable problem.
Nucleic acids such as DNA and RNA are based on a sugar-polymer backbone. Again, the presence of some sugars that are mono-functional would terminate the formation of these and the presence of water also drives this reaction in the wrong direction as well (to fall apart). Also, like amino acids, nucleotides can join in the wrong way without enzymes to direct them; yet another insurmountable problem.

The origin of life is a matter of programming, not just chemistry
The above information would be sufficient to eliminate notions of the naturalistic origin of life, but we have not covered the most important problem, which is the origin of the programming. Life is not based just on polymers but polymers with specific arrangements of the subunits; specific arrangements of amino acids to make functional proteins/enzymes and specific arrangements of nucleic acid bases to make functional DNA and RNA.
As astrobiologist Paul Davies, of the Beyond Center for Fundamental Concepts in Science at Arizona State University, said,
“To explain how life began we need to understand how its unique management of information came about.
“The way life manages information involves a logical structure that differs fundamentally from mere complex chemistry. Therefore chemistry alone will not explain life’s origin, any more than a study of silicon, copper and plastic will explain how a computer can execute a program.”19
Davies’ clarity on this point ought not to be a surprise to his fellow evolutionists, given his similarly plain-speaking public utterances for well over a decade previously. E.g. “It is the software of the living cell that is the real mystery, not the hardware.” And: “How did stupid atoms spontaneously write their own software? … Nobody knows …”.20
Any attempt to explain the origin of life without explaining the origin of the information processing system and the information recorded on the DNA of a living cell is avoiding the issue. We just have to look at the simplest free-living cell possible to see how the origin of the information is an insoluble problem for scenarios that rely on physics and chemistry (that is, no intelligent design allowed).
Sir Karl Popper, one of the most prominent philosophers of science of the 20th century, realized that,
“What makes the origin of life and of the genetic code a disturbing riddle is this: the genetic code is without any biological function unless it is translated; that is, unless it leads to the synthesis of the proteins whose structure is laid down by the code. But … the machinery by which the cell (at least the non-primitive cell, which is the only one we know) translates the code consists of at least fifty macromolecular components which are themselves coded in the DNA [ed: we now know that over 100 macromolecular components are needed]. Thus the code cannot be translated except by using certain products of its translation. This constitutes a baffling circle; a really vicious circle, it seems, for any attempt to form a model or theory of the genesis of the genetic code.
“Thus we may be faced with the possibility that the origin of life (like the origin of physics) becomes an impenetrable barrier to science, and a residue to all attempts to reduce biology to chemistry and physics.”21
Origin of the DNA code
The coded DNA information storage system as described by Popper cannot arise from chemistry, but demands an intelligent cause.22 If we think of other coding systems, such as the Morse code or a written alphabetical language, where symbols were invented to represent the sounds of speech, such coded systems only arise from intelligence. It is an arbitrary convention that ‘a’ is usually pronounced as in ‘cat’ in English; nothing about the shape of the letter indicates how it should be pronounced. Likewise, there is just no conceivable possibility of explaining the DNA coding system from the laws of physics and chemistry because there is no physical or chemical relationship between the code and what is coded.
Furthermore, if the origin of any DNA code were not a big enough problem, the DNA code turns out to be, of the many millions possible, “at or very close to a global optimum for error minimization: the best of all possible codes.”23 This error minimization in the code is possible because there are potentially 64 ‘codons’24 for 20 amino acids, so that nearly all amino acids have more than one codon (a few common amino acids, such as leucine, have six).25 These multiple codons are sometimes called ‘redundant’, often taken to mean ‘extra to needed’ or ‘superfluous’. However, the extra codons are optimized such that the most likely single-letter mistakes (mutations) in the coding are more likely not to change the amino acid, or at least to change it to a chemically similar one (thus being less disruptive to the structure of the protein manufactured).
The extra codons are also involved in sophisticated control of the amount of protein synthesized, through ‘translation level control’. This control system operates in bacteria and higher organisms.26 (See below for more on gene regulation.)
There is no way that a coding system can develop in successive stages to be optimized. If any workable coding system did come into existence by some incredible fluke, no significant change in the basic code could thenceforth occur because the code and the decoding system (reading machinery) would have to change at the same time (there are some very minor variations in the basic code in some bacteria, for example, where one of the three normal ‘stop’ codons codes for an extra amino acid to the normal 20). So the optimized code cannot be explained except as another incredible fluke of ‘nature’, right at the supposed beginning of life.
Not just a coding system, but information
Not only does the origin of the coded information storing system need to be explained, the information or specifications for proteins, etc., stored on the DNA has also to be explained. Revisiting the simplest cell, derived by knocking out genes from a viable free-living microbe to see which ones were ‘essential’, this minimal cell needs over 400 protein and RNA components. Specifications for all these have to be encoded on the DNA, otherwise this hypothetical cell cannot manufacture them or reproduce itself to make another cell. It would take a large book to print this information coded in the four ‘letters’ of the DNA.
As per the Paul Davies analogy, the problem is similar to a computer program. How do we explain the existence of a program? There is first the programming language (Python, Fortran, C++, Basic, Java, etc.) but then there is the actual set of instructions written in that language. The DNA problem is likewise two-fold; the origin of the programming language and the origin of the program.
Proposals for something simpler that ‘evolved’ into this simplest cell need to demonstrate the route from their hypothetical simpler start to the first living cell. Enthusiasts for abiogenesis often appeal to ‘billions of years’ as a hand-waving approach to solving the problems, but this provides no mechanism. Reactions that are going in the wrong direction are not going to reverse and go in the correct direction by adding more time.
Gene regulation is necessary
Genes in viable living cells are regulated; the cell controls the activity of each gene to match the cell’s needs.
Production of proteins, such as enzymes to digest food, requires energy as well as the raw ingredients (amino acids). If a gene is unregulated, the protein it codes for will be produced up to the limit of the energy and/or amino acid supply; whichever is exhausted first. Multiply this by the 400+ genes in the minimal viable free-living cell and you have ultra-chaos and non-viability. A lack of gene regulation would obviously be unworkable.
Furthermore, many products of enzyme-catalysed reactions are toxic if manufactured in too great a quantity (‘the dose makes the poison’). For example, Down Syndrome problems are caused by overexpression of genes on the tripled chromosome 21.
It seems that all processes in cells are regulated and in very precise ways. Not surprisingly, failure of individual gene regulation causes many diseases in humans (doi: 10.1016/j.cell.2013.02.014).
Gene regulation involves sensing a need and producing the right amounts of proteins to meet that need.
A major form of gene regulation involves proteins that bind to a gene or a DNA sequence that activates a gene or suite of genes. Binding of the protein can stop the production of the protein(s) or promote it. Binding or not-binding can be determined by the presence of a substrate or a product of the reactions that are catalysed by the protein/enzyme(s) produced. Thus, the amount of enzyme produced matches the need.
Another form of gene regulation involves methylation. This entails the addition of methyl (-CH3) groups to some of the nucleic acid bases that make up a gene. This then influences the activity of the gene (whether it is transcribed to mRNA and produces a protein or not). When the J. Craig Venter Institute produced their ‘synthetic’ cell, Syntia, in 2010, they found that it was not viable unless they copied the DNA methylation pattern of the bacterium that they were copying (Mycoplasma mycoides).
There are many types of gene regulation, even in the simplest living single cells. How could gene regulation arise, as it would need to be present when a new gene came into existence? Without regulation a new gene would create chaos. The origin of any new gene is difficult enough, but the need to add its regulatory network at the same time adds further to the impossibility of abiogenesis.
Life also needs error-correcting systems
Molecular biology has revealed that cells are phenomenally complex and sophisticated, even the simplest ones. The information, as stated, is stored on the DNA. However, DNA is an unstable molecule. One report says:
“There is a general belief that DNA is ‘rock solid’—extremely stable”, says Brandt Eichman, associate professor of biological sciences at Vanderbilt, who directed the project. “Actually DNA is highly reactive. On a good day about one million bases in the DNA in a human cell are damaged.”27
Therefore all cells must have systems for correcting faults that develop in the structure of the DNA or in the coded information. Without these error-correcting systems, the number of errors in the DNA sequence accumulate and result in the demise of the cell (‘error catastrophe’). This feature of all living cells adds yet another ‘impossible’ to origin of life scenarios.
Any information that happened to arise on a theoretical DNA molecule in a primordial soup would have to be reproduced accurately or the information would be lost due to copying errors and chemical damage. Without an already functioning repair mechanism, the information would be degraded quickly. However, the instructions to build this repair machinery are encoded on the very molecule it repairs, another vicious circle for origin-of-life scenarios.28
When scientists discovered bacteria that live in extreme conditions, such as around hydrothermal vents in the sea, they were heralded as ‘primitive life’ because some origin-of-life researchers had proposed that life might have started in such places. However, these ‘extremophiles’, as they have been called (‘liking extremes’), have quite sophisticated error-correcting systems for their DNA. For example, Deinococcus radiodurans is a bacterium that can withstand extreme doses of ionizing radiation that would kill you or me, or other bacteria. It does sustain DNA damage where the DNA is fractured into many pieces. However, about 60 genes are activated to repair the breaks and reconstruct the genome in the hours following the damage.29
Hydrothermal vents are hot, inhospitable places and the DNA of microbes that live there is continually being damaged, such that the microbes must have sophisticated error-protecting and correcting systems to survive. They are not at all simple and do not provide any sort of viable model for explaining the origin of life.30
Moreover, all bacteria, not just the ‘extremophiles’, must have sophisticated error-correcting systems that involve many genes, and when the error correction is inactivated by mutations the bacteria become non-viable. This provides yet another problem for the origin of life.
Origin-of-life scenarios
Did life originate in a warm pond (as speculated by Darwin), near a deep sea vent, on clay particles, or somehow/somewhere else? The number of scenarios proposed, with no winner, suggests that they all have major deficiencies.
A major problem with warm pond and deep sea vent ideas is the presence of water, which prevents many of the reactions needed; to get polymers, for example. Furthermore, the heat in deep sea vents would speed up the breakdown of any lucky chemical formation.
Because of these problems with the presence of water, physical chemist and origin-of-life researcher, Graham Cairns-Smith proposed that clay surfaces were involved in facilitating some of the needed reactions.
However, experiments in warm volcanic ponds have shown that clay particles bind amino acids, DNA and phosphate, essential components of life, so strongly that the clay prevents any necessary reactions from occurring.31
The origin of a whole cell including the DNA, proteins and RNA needed for it to reproduce will never happen by an accident in a chemical soup, as demonstrated above. So advocates of abiogenesis have tried to imagine scenarios whereby life began with simpler requirements and then progressed to life as we know it today.
Proteins first?
Most effort has gone into a ‘proteins first’ approach, whereby proteins supposedly formed first and the DNA sequences to make the needed proteins and the RNAs necessary to make proteins from the sequences of DNA came later. However, other than the problem of getting the correct set of optically-pure amino acids and the problem of polymerisation to make the protein chains of amino acids, few proteins can act as templates to make copies of themselves.32 Also, a fundamental problem is that there is no mechanism for creating the DNA sequence for a protein from the protein itself, as pointed out by information theorist Hubert Yockey.33
RNA first?
In the 1980s, some RNA molecules were discovered that have the ability to catalyse some chemical reactions; these were dubbed ‘ribozymes’ (from ribonucleic acid enzymes). This finding stimulated a lot of excitement and so a lot of effort has gone into RNA-first scenarios, or the ‘RNA world’. At least there are enzymes that can generate DNA code from RNA code; that is, if you could get the RNA you might be able to imagine a scenario for getting the DNA. However, the enzyme complexes that can make a DNA copy of an RNA sequence are phenomenally complex and themselves would never arise by natural processes. And there are many other seemingly insurmountable problems with the RNA-first scenarios, 19 of which have been enumerated by Cairns-Smith.34 Furthermore, RNA is much less stable than DNA, which itself is very unstable, as documented above.
The multiplicity of scenarios proposed reinforces the conclusion that researchers really have little idea how life could have ‘made itself’. There is no viable hypothesis as to how life could start off simpler and, step-wise, progress to become an actual living cell. Neo-Darwinism (mutations and natural selection) is often invoked to try to ‘climb mount impossible’ but this cannot help, even hypothetically, until there is a viable self-reproducing entity, aka a cell, the minimum requirements for which I set out earlier (‘What are the minimum requirements for a cell to live?’).
Life from outer space?
Francis Crick, co-discoverer of the DNA double helix structure, is a well-known proponent of ‘life from space’.35 He proposed that aliens sent life to Earth, known as ‘directed panspermia’. Another form of this idea, simply ‘panspermia’, is that life arose somewhere else in the universe and came to Earth as microbes on meteorites or comets; Earth was ‘seeded’ with life in this manner. Either version of panspermia effectively puts the matter beyond the reach of science. About the only element of panspermia that is testable is the ability of microbes to survive riding on/in a meteorite to Earth. And this has been tested and found wanting; microbes don’t survive.36
A lot of the impetus for the search for extra-terrestrial intelligence (SETI) and extra-solar planets comes from a desire to find evidence that life might have formed ‘out there’. But even allowing the whole universe as a laboratory does not solve the problem; life would never form, as the following section reinforces.
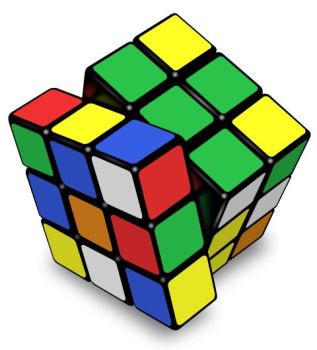
Probability calculations for the origin of life
Many attempts have been made to calculate the probability of the formation of life from chemicals, but all of them involve making simplifying assumptions that make the origin of life even possible (i.e. probability > 0).
Mathematician Sir Fred Hoyle stated in various ways the extreme improbability of life forming, or even getting a single functional biopolymer such as a protein. Hoyle said, “Now imagine 1050 blind persons [ed: standing shoulder to shoulder, they would more than fill our entire planetary system] each with a scrambled Rubik cube and try to conceive of the chance of them all simultaneously arriving at the solved form. You then have the chance of arriving by random shuffling of just one of the many biopolymers on which life depends. The notion that not only the biopolymers but the operating program of a living cell could be arrived at by chance in a primordial soup here on Earth is evidently nonsense of a high order. Life must plainly be a cosmic phenomenon.”37
Indeed, we can calculate the probability of getting just one small protein of 150 amino acids in length, assuming that only the correct amino acids are present, and assuming that they will join together in the right manner (polymerize). The number of possible arrangements of 150 amino acids, given 20 different ones, is (20)150. Or the probability of getting it right with one try is about 1 in 10195. Lest someone protest that not every amino acid has to be in the exact order, this is only a small protein, and only one of several hundred proteins needed, many of which are much larger, and the DNA sequence has to arise as well, seriously compounding the problem. Indeed there are proteins that will not function at all with even a small alteration to their sequence.38
At that time Hoyle argued that life must therefore have come from outer space. Later he realized that even given the universe as a laboratory, life would not form anywhere by the unguided (non-intelligent) processes of physics and chemistry:
“The likelihood of the formation of life from inanimate matter is one to a number with 40,000 naughts after it … It is big enough to bury Darwin and the whole theory of evolution. There was no primeval soup, neither on this planet nor any other, and if the beginnings of life were not random, they must therefore have been the product of purposeful intelligence.”39
Does a figure of 1 in 1040,000 make the origin of life somewhere in the universe impossible without purposeful intelligence? Can we say that?
The total number of events (or ‘elementary logical operations’) that could have occurred in the universe since the supposed big bang (13.7 billion years) has been calculated at no more than 10120 by MIT researcher Seth Lloyd.40 This sets an upper limit on the number of experiments that are theoretically possible. This limit means that an event with a probability of 1 in 1040,000 would never happen. Not even our one small protein of 150 amino acids would form.
However, biophysicist Harold Morowitz41 came up with a much lower probability of 1 in 1010,000,000,000. This was the chance of a minimalist bacterium being assembled from a broth of all the basic building blocks (e.g. theoretically obtained by heating a brew of living bacteria to kill them and break them down to their basic constituents).
As an atheist, Morowitz argued that therefore life was not a result of chance and posited that there must be some property of available energy that drives the formation of entities that can use it (aka ‘life’). This sounds much like the idea of Gaia, which attributes pantheistic mystical properties to the universe.
The atheist philosopher Thomas Nagel proposed something similar to account for the origin of life and mind.42
Anything but believe in a supernatural Creator, it would appear.
The different probabilities calculated arise from the difficulty of calculating such probabilities and the differing assumptions that are made. If we make calculations using assumptions that are most favourable to abiogenesis and the result is still ridiculously improbable, then it is a more powerful argument than using more realistic assumptions that result in an even more improbable result for the materialist (because the materialist can try to argue against some of the assumptions with the latter approach).
However, all calculations of the probability of the chemical origin of life make unrealistic assumptions in favour of it happening, otherwise the probability would be zero. For example, Morowitz’s broth of all the ingredients of a living cell cannot exist because the chemical components will react with each other in ways that will render them unavailable for forming the complex polymers of a living cell, as explained above.
High profile information theorist Hubert Yockey (UC Berkeley) realized this problem:
“The origin of life by chance in a primeval soup is impossible in probability in the same way that a perpetual motion machine is in probability. The extremely small probabilities calculated in this chapter are not discouraging to true believers … [however] A practical person must conclude that life didn’t happen by chance.”43
Note that in his calculations, Yockey generously granted that the raw materials were available in a primeval soup. But in the previous chapter of his book, Yockey showed that a primeval soup could never have existed, so belief in it is an act of ‘faith’. He later concluded, “the primeval soup paradigm is self-deception based on the ideology of its champions.”44
More admissions
Note that Yockey is not the only high-profile academic to speak plainly on this issue:
“Anyone who tells you that he or she knows how life started on Earth some 3.4 billion years ago is a fool or a knave. Nobody knows.”—Professor Stuart Kauffman, origin of life researcher, University of Calgary, Canada.45
“…we must concede that there are presently no detailed Darwinian accounts of the evolution of any biochemical or cellular system, only a variety of wishful speculations.” —Franklin M. Harold, Emeritus Professor of Biochemistry and Molecular Biology Colorado State University.46
“We are almost as much in the dark today about the pathway from nonlife to life as Charles Darwin was when he wrote, ‘It is mere rubbish thinking at present of the origin of life; one might as well think of the origin of matter.’”—Paul Davies, director of BEYOND: Center for Fundamental Concepts in Science at Arizona State University.47
“The novelty and complexity of the cell is so far beyond anything inanimate in the world today that we are left baffled by how it was achieved.”— Kirschner, M.W. (professor and chair, department of systems biology, Harvard Medical School, USA), and Gerhart, J.C. (professor in the Graduate School, University of California, USA).48
“Conclusion: The scientific problem of the origin of life can be characterized as the problem of finding the chemical mechanism that led all the way from the inception of the first autocatalytic reproduction cycle to the last common ancestor. All present theories fall far short of this task. While we still do not understand this mechanism, we now have a grasp of the magnitude of the problem.”49
“The biggest gap in evolutionary theory remains the origin of life itself… the gap between such a collection of molecules [amino acids and RNA] and even the most primitive cell remains enormous.”—Chris Wills, professor of biology at the University of California, USA.50
Even the doctrinaire materialist Richard Dawkins admitted to Ben Stein (Expelled, the movie documentary) that no one knows how life began:
Richard Dawkins: “We know the sort of event that must have happened for the origin of life—it was the origin of the first self-replicating molecule.”
Ben Stein: “How did that happen?”
Richard Dawkins: “I’ve told you, we don’t know.”
Ben Stein: “So you have no idea how it started?”
Richard Dawkins: “No, nor has anybody.”51
“We will never know how life first appeared. However, the study of the appearance of life is a mature, well-established field of scientific inquiry. As in other areas of evolutionary biology, answers to questions on the origin and nature of the first life forms can only be regarded as inquiring and explanatory rather than definitive and conclusive.”52 [emphasis added]

The James Tour challenge
In August 2023, Rice University organic chemist, Dr James Tour, issued a 60-day challenge to origin-of-life researchers. Tour offered to remove all his videos on the topic if three leading experts agreed that any of five fundamental problems had been solved:
- Linking of amino acids into chains (proteins)
- Linking of nucleotides into RNA molecules
- Linking of simple sugars into chains (polysaccharides)
- Origin of biological information
- Assembly of components into a cell
The deadline expired without any solutions offered to any of these problems.53
Conclusion
Life did not arise by physics and chemistry without intelligence. The intelligence needed to create life, even the simplest life, is far greater than that of humans; we are still scratching around trying to understand fully how the simplest life forms work. There is much yet to be learned of even the simplest bacterium. Indeed, as we learn more, the ‘problem’ of the origin of life gets more difficult; a solution does not get nearer, it gets further away. But the real problem is this: the origin of life screams at us that there is a super-intelligent Creator of life and that is just not acceptable to the secular mind of today.
The origin of life is about as good as it gets in terms of scientific ‘proof’ for the existence of God.
Re-featured on homepage: 13 February 2024
References and notes
- http://evolution.berkeley.edu/evosite/evo101/IIE2aOriginoflife.shtml (accessed 17 October 2013). Return to text.
- Myers, P.Z., 15 misconceptions about evolution, 20 February 2008, scienceblogs.com; Matzke, N., What critics of neo-creationists get wrong: a reply to Gordy Slack, pandasthumb.org. Dawkins tries to deal with the origin of life in his book The Greatest Show on Earth, where he claims to ‘prove evolution’. See Sarfati, J., The Greatest Hoax on Earth? ch. 13, 2010, Creation Book Publishers. Return to text.
- Kerkut, G.A., Implications of Evolution, Pergamon, Oxford, UK, p. 157, 1960 (available online at ia600409.us.archive.org/23/items/implicationsofev00kerk/implicationsofev00kerk.pdf); creation.com/evolution-definition-kerkut. Return to text.
- Sugars have linear forms that contain carbonyls—see Fig. 2. The cyclic forms that occur in nucleic acids also predominate in solution form, but in equilibrium with the linear form. When something reacts strongly with the aldehyde, more of the linear form is regenerated to replace that which is reacted, so all the sugar molecules will be consumed. Return to text.
- Sarfati, J., World record enzymes, Journal of Creation 19(2):13–14, 2005; creation.com/world-record-enzymes-richard-wolfenden. Return to text.
- Bergman, J., Why the Miller-Urey research argues against abiogenesis. Return to text.
- Truman, R., What biology textbooks never told you about evolution. Return to text.
- Sarfati, J., Origin of life: instability of building blocks. Return to text.
- Chadwick, A.V., Abiogenic Origin of Life: A Theory in Crisis, 2005; origins.swau.edu/papers/life/chadwick/default.html. Return to text.
- See for example Potassium ion channel, hydrated ionic radii, creation.com/ionic-error, 21 August 2010. Return to text.
- Krogh, A. et al., Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes, Journal of Molecular Biology 305(3):567–580, 2001; dx.doi.org/10.1006/jmbi.2000.4315. Return to text.
- Transporter Proteins in Mycoplasma genitalium G-37; membranetransport.org/index.html (accessed 11 Oct. 2013). Return to text.
- The ‘right’ and ‘left’ in terms of chirality refer to the position of the amino group (NH2) as displayed on a standardized diagram (Fischer projection) of an amino acid. Return to text.
- Sarfati, J., Origin of life: the chirality problem; creation.com/origin-of-life-the-chirality-problem (updated 2010). Return to text.
- Cairns-Smith, A.G., Evolutionist criticisms of the RNA World conjecture, from Genetic Takeover and the Origin of Life, 1982; creation.com/cairns-smith-detailed-criticisms-of-the-rna-world-hypothesis. Return to text.
- Stanford researchers produce first complete computer model of an organism; news.stanford.edu, 19 July 2012. Return to text.
- Sarfati, J., How simple can life be? https://creation.com/how-simple-can-life-be. Research on a synthetic cell, JCVI-syn3.0, showed that 473 genes were essential, 65 of which had no known function: C.A. Hutchison III et al., Design and synthesis of a minimal bacterial genome, Science 351:1414, March 25, 2016; doi: 10.1126/science.aad6253. Return to text.
- Sarfati, J., Origin of life: the polymerization problem. Return to text.
- Davies, P., The secret of life won’t be cooked up in a chemistry lab: Life’s origins may only be explained through a study of its unique management of information, The Guardian, Sunday 13 January 2013; guardian.co.uk/commentisfree/2013/jan/13/secret-life-unveiled-chemistry-lab. Return to text.
- Davies, P., Life force, New Scientist 163(2204):27–30, September 18, 1999. Return to text.
- Popper, K.R., “Scientific reduction and the essential incompleteness of all science”; in Ayala, F. and Dobzhansky, T., (Eds.)., Studies in the Philosophy of Biology, University of California Press, Berkeley, p. 270, 1974. Return to text.
- Smith, C., Lost in translation: The genetic information code points to an intelligent source, 6 May 2010; creation.com/genetic-code-intelligence. Return to text.
- Freeland, S.J., et al., Early fixation of an optimal genetic code, Molecular Biology and Evolution 17(4):511–18, 2000; mbe.oxfordjournals.org/content/17/4/511.full. Return to text.
- With four nucleotide ‘letters’ comprising DNA and with three read at a time (a ‘codon’) by the reading machinery, this gives 4x4x4=64 different possibilities (3-letter ‘codons’). Return to text.
- Three are usually used as ‘stop’ codes to mark the end of a protein coding sequence, so 61 are normally used for amino acid coding. Return to text.
- Novoa, E.M. and de Pouplana, L.R., Speeding with control: codon usage, tRNAs, and ribosomes, Trends in Genetics 28(11):574–581, November 2012; ww2.biol.sc.edu/~elygen/biol655/translation%20speed.pdf. Return to text.
- Newly discovered DNA repair mechanism, Science News, sciencedaily.com, 5 October 2010. Return to text.
- Sarfati, J., New DNA repair enzyme discovered, 13 January 2010; creation.com/DNA-repair-enzyme. Tomas Lindahl, Paul Modrich, and Aziz Sancar won the 2015 Nobel Prize for Chemistry for discovering three different DNA repair mechanisms: Batten, D., DNA repair mechanisms ‘shout’ creation, Creation 38(2):56, April 2016. Return to text.
- Cox, M.M., Keck, J.L. and Battista, J.R., Rising from the Ashes: DNA Repair in Deinococcus radiodurans, PLoS Genetics 6(1): e1000815, 2010; doi:10.1371/journal.pgen.1000815. Return to text.
- Catchpoole, D., Life at the extremes, Creation 24(1):40–44, 2001; creation.com/extreme and Sarfati, J., Hydrothermal origin of life? Journal of Creation 13(2):5–6, 1999; creation.com/hydrothermal. Return to text.
- Morelle, R., Darwin’s warm pond idea is tested, 13 Feb. 2006; news.bbc.co.uk/2/hi/science/nature/4702336.stm. Return to text.
- Prions are sometimes proposed as replicating proteins, but prions cause existing proteins to become misshapen; they don’t replicate themselves by causing amino acids to line up in the correct sequence to make a copy of the prion (prions are thought to cause ‘mad cow disease’). Return to text.
- Yockey, H., Information Theory, Evolution and the Origin of Life, Cambridge University Press, 2005, pp. 118–119. Return to text.
- Evolutionist criticisms of the RNA World conjecture; Quotable Quote by Cairns-Smith; creation.com/cairns-smith-detailed-criticisms-of-the-rna-world-hypothesis. See also, Mills, G.C. and Kenyon, D., The RNA World: A Critique, Origins & Design 17(1); arn.org/docs/odesign/od171/rnaworld171.htm. Return to text.
- Bates, G., Designed by aliens? Creation 25(4):54–55, 2003; creation.com/aliens. Return to text.
- Sarfati, J., Panspermia theory burned to a crisp: bacteria couldn’t survive on meteorite, 10 Oct 2008; creation.com/panspermia-theory-burned-to-a-crisp-bacteria-couldnt-survive-on-meteorite. Return to text.
- Hoyle, Fred, The Big Bang in Astronomy, New Scientist 92:521–527, 1981. Return to text.
- For example, Royal Truman has researched the protein ubiquitin, present in eukaryotes, to show that little variation in the sequence is permitted for functionality, so that the chance (naturalistic) origin of such a protein is ruled out; see Truman, R., The ubiquitin protein: chance or design? Journal of Creation 19(3):116–127, 2005; creation.com/the-ubiquitin-protein-chance-or-design. Return to text.
- Sir Fred Hoyle, as quoted by Lee Elliot Major, “Big enough to bury Darwin”. Guardian (UK) education supplement, Thursday August 23, 2001; education.guardian.co.uk/higher/physicalscience/story/0,9836,541468,00.html. Return to text.
- Lloyd, Seth, Computational capacity of the universe, Physics Review Letters 88:237901, 2002; http://arxiv.org/abs/quant-ph/0110141v1. Return to text.
- Morowitz, H., Energy Flow in Biology, Academic Press, NY, 1968. Return to text.
- Nagel, T., Mind and Cosmos: Why the Materialist Neo-Darwinian Conception of Nature Is Almost Certainly False, Oxford University Press, 2012. Return to text.
- Yockey, H., Information Theory and Molecular Biology, Cambridge University Press, 1992, p. 257. Return to text.
- Ibid. p. 336; see Quotable quote: Primeval soup—failed paradigm. Return to text.
- Stuart Kauffman, At Home in the Universe: The Search for the Laws of Self Organization and Complexity, Oxford University Press, p. 31, 1995. Return to text.
- Harold, F.M., The way of the cell: molecules, organisms and the order of life, Oxford Uni. Press, New York, p. 205, 2001. Return to text.
- Davies, Paul, The Cosmos Might Be Mostly Devoid of Life: We still have no idea how easy it is for life to arise—and it may be incredibly difficult, Scientific American, 1 September 2016; www.scientificamerican.com/article/the-cosmos-might-be-mostly-devoid-of-life. Return to text.
- Kirschner, M.W. and Gerhart, J.C., The plausibility of life: Resolving Darwin’s Dilemma, Yale University Press, New Haven and London, p. 256, 2005. Return to text.
- Watchershauser, G., Origin of life: RNA world versus autocatalytic anabolism, The Prokaryotes, Vol. 1, 3rd edition, chapter 1.11, pp. 275–283, p. 282, 2006. Return to text.
- Quoted in, Evolution’s final frontiers, New Scientist 201(2693):42, 2009. Return to text.
- Expelled: no intelligence allowed, Premise Films, 2008. Return to text.
- Lazcano, Antonio, Historical Development of Origins Research, Cold Spring Harbor Perspectives in Biology 2(11): a002089, November 2010; doi: 10.1101/cshperspect.a002089. Return to text.
- Miller, B., On origin of life, chemist James Tour has successfully called these researchers’ bluff, Evolution News 31 Oct. 2023; evolutionnews.org. Return to text.






Readers’ comments
Comments are automatically closed 14 days after publication.