How cells handle heme
A poison that’s vital to life!

The chances are, you will remember learning about hemoglobin (or haemoglobin in British spelling). This is the pigmented protein that gives your red blood cells their colour. It is responsible for picking up oxygen in the lungs, then carrying it to every cell of your body. As we will see shortly, recent discoveries concerning its central component, the red pigment heme (or haem), pose real problems for evolutionary theory.
Too much heme is actually toxic to cells, yet it is literally vital for life on our planet. This is true not only for human beings, but for virtually all animals and plants—and even for more lowly organisms like bacteria, yeast, and fungi. Manufacturing it is therefore an absolutely essential biological process, but this must be tightly controlled.
A multi-purpose molecule
Several variations on the heme molecule exist in living things, but their basic structure is the same: they combine a square-shaped construction called a porphyrin ring with iron (see figure 1).
Hemoglobin is one of many different kinds of ‘hemoprotein’ in which heme with its iron is bound to a protein component. Hence these are all types of what are called ‘metalloproteins’. Other types of metalloproteins incorporate different metals, including copper, zinc, and cobalt—and magnesium in the chlorophyll of green plants. (Chlorophyll is essential for photosynthesis, in which energy-rich foods are made from sunlight, water and carbon dioxide).
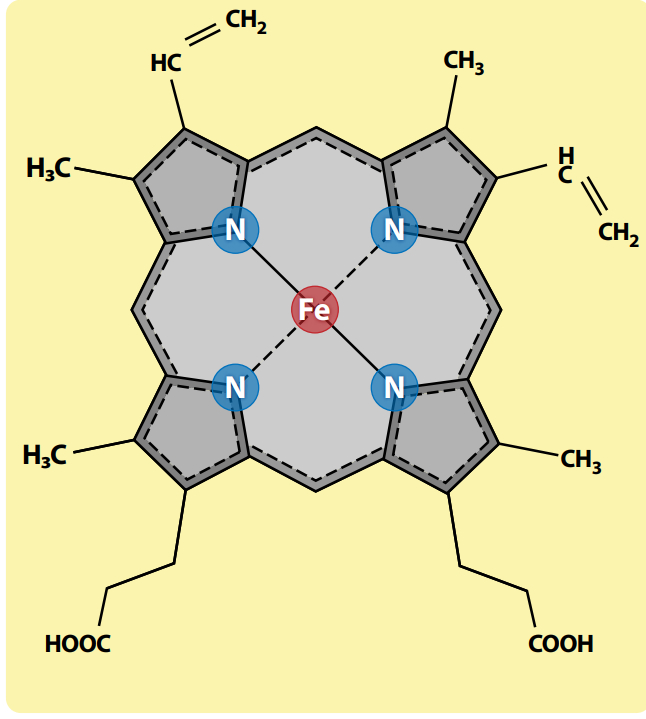
Think of heme as a versatile cellular gizmo, with all sorts of regulating and signalling roles inside cells. As well as its crucial role within hemoglobin, it is a key component of:
- other pigmented proteins (e.g. myoglobin in your muscles, giving them their red colour);
- a variety of peroxidase enzymes; and
- cytochromes, proteins involved in major metabolic pathways in your cells.
Recent research also suggests that heme helps regulate the activity of your cells’ powerhouses, the mitochondria. It seems that the heme directly influences the amount of ATP (adenosine triphosphate) made within these mitochondrial energy factories.1 ATP is the ‘fuel’, which powers living things, and making it is famously the job of one of God’s majestic molecular motors.2
Supply on demand
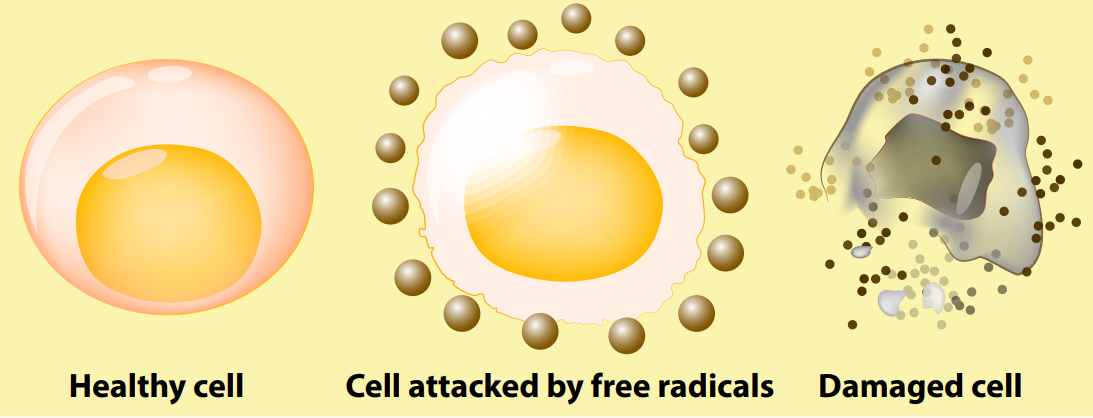
The authors of a recent paper in the leading journal PNAS describe how this talented-but-tricky heme molecule is manufactured, then mobilized in just the right amounts, when and where required.3 Superfine control is a must, because too much heme would result in what are called free radicals. These are short-lived but super-reactive atoms or molecules which damage membranes, DNA and other parts of cells. Left unchecked, an overload of free radicals contributes to cancer, heart and blood vessel problems, all sorts of degenerative diseases, and ageing.4
Free radicals are actually a byproduct of your everyday metabolism. But they also result from exposure to environmental toxins (like tobacco smoke) as well as to other cancer-causing substances, and to radiation (e.g. ultraviolet light). Various powerful antioxidant molecules are therefore important because they can destroy these free radicals—hence are called free radical scavengers. Some of these are found in your body, and a healthy diet furnishes you with many antioxidants too.5
Interestingly, the researchers found that, at any one time, there is no more than a single heme molecule in any of your cells’ compartments.4 We’ve seen that despite being essential, heme is rather ‘hot to handle’. So how do cells maintain sufficient quantities of it, given the need for immediate supply on demand? Stockpiling such material is dangerous (because of the free radical risk). So one might guess the answer must involve super-streamlined manufacture, coupled with a super-efficient distribution system. In fact, the research team’s experiments point instead to a clever ‘buffering’6 system; since ‘free’ (unbound) heme is toxic, most of the available heme molecules are weakly (reversibly) bound to special ‘buffer’ proteins so they can be readily given up when required.
This explains the minuscule amount of free heme ascertained in this PNAS research. Instead, cells harbour pools of this ‘labile heme’ (so-called because it is ‘unstable’ in the sense of being readily capable of undergoing change to the free heme). The paper’s authors describe this as “a supply of exchangeable heme, to be made available on demand in this highly controlled fashion …” They add: “This exquisite control also provides a mechanism for heme-dependent signaling and regulation, as heme can be supplied discretely, leading to the switching on of proteins in single-molecule steps.”3
Dynamic design
It’s clever stuff! This is yet another demonstration of the fact that cells are far from simple. Here we see they are logistics experts, employing complex systems of checks and balances. In this case, it involves perfectly gauging the supply and demand of this absolutely essential but potentially deadly ‘commodity’—heme.
Did you notice the language used by the scientists—“highly controlled”, “exquisite control”, “signaling and regulation”? It is the language of design. Of course it is! As even the most ardent atheists know in their hearts, such elegant systems have never been shown to originate without the input of intelligent and purposeful agents. Unsurprisingly, it is when such systems begin to malfunction (in our fallen world) that various diseases inevitably occur.
How could such superlative control have evolved step-by-tiny-step, without any guiding hand? And with no plan or purpose, as advocates of evolution must insist? There are no less than eight enzymatic steps in the complex chemical pathway for the making of heme, some of which occur in your marvellous mitochondria, and some within the cytoplasm of your cells. Heme manufacture is anything but simple!7
And recall that heme is found in practically all living things. For the naturalistic origin of cellular life to be viable, evolutionists would need to first demonstrate how those steps might have arisen through random mutations. And they must believe that these arose at a ‘very early’ proto-cell stage.8
Such imaginative, godless schemes are really nothing short of expecting miracles from lifeless nature. Heme manufacture and handling in your cells are marvels that demand we acknowledge our Creator God, for it is He “who does great things and unsearchable, marvellous things without number” (Job 5:9).
References and notes
- Li, Y. and 13 others, MFSD7C switches mitochondrial ATP synthesis to thermogenesis in response to heme, Nature Communications 11(1):7837, 24 Sep 2020. Return to text.
- Thomas, B., ATP synthase: majestic molecular machine made by a mastermind, Creation 31(4):21–23, 2009; creation.com/atp-synthase. Return to text.
- Leung, G.C.-H. and 6 others, Unravelling the mechanisms controlling heme supply and demand, PNAS 118(22):e2104008118, 1 Jun 2021. Return to text.
- Pham-Huy, L.A., and 2 others, Free radicals, antioxidants in disease and health, Int. J. Biomed. Sci. 4(2):89–96, 2008. Return to text.
- Examples of antioxidants in the body are glutathione and alpha lipoic acid, but many foods (fruits, vegetables, whole grains, nuts, herbs, spices, and more) plus drinks (e.g. green tea, herbal teas, fruit juice, etc.) are significant sources of antioxidants. Vitamin C is a well-known antioxidant. Return to text.
- In computing, a buffer is an area of temporary storage of data while it is being transferred from A to B, e.g. in print spooling. Return to text.
- Phillips, J.D., Heme biosynthesis and the porphyrias, Mol. Genet. Metab. 128(3):164–177, 22 Apr 2019. Return to text.
- “Designed for [a] purpose”—heme production defeats evolution, evolutionnews.org, 28 Jun 2021. Return to text.






Readers’ comments
Comments are automatically closed 14 days after publication.