Journal of Creation 10(3):335–343, December 1996
Browse our latest digital issue Subscribe
Excess argon within mineral concentrates from the new dacite lava dome at Mount St Helens volcano
Summary
The conventional K-Ar dating method was applied to the 1986 dacite flow from the new lava dome at Mount St Helens, Washington. Porphyritic dacite which solidified on the surface of the lava dome in 1986 gives a whole rock K-Ar ‘age’ of 0.35 ± 0.05 million years (Ma). Mineral concentrates from the dacite which formed in 1986 give K-Ar ‘ages’ from 0.34 ± 0.06 Ma (feldspar-glass concentrate) to 2.8 ± 0.6 Ma (pyroxene concentrate). These ‘ages’ are, of course, preposterous. The fundamental dating assumption (‘no radiogenic argon was present when the rock formed’) is questioned by these data. Instead, data from this Mount St Helens dacite argue that significant ‘excess argon’ was present when the lava solidified in 1986. Phenocrysts of orthopyroxene, hornblende and plagioclase are interpreted to have occluded argon within their mineral structures deep in the magma chamber and to have retained this argon after emplacement and solidification of the dacite. The amount of argon occluded is probably a function of the argon pressure when mineral crystallization occurred at depth and/or the tightness of the mineral structure. Orthopyroxene retains the most argon, followed by hornblende, and finally, plagioclase. The lava dome at Mount St Helens dates very much older than its true age because phenocryst minerals inherit argon from the magma. The study of this Mount St Helens dacite causes the more fundamental question to be asked—how accurate are K-Ar ‘ages’ from the many other phenocryst-containing lava flows worldwide?
Introduction

Dacite magma at Mount St Helens in Washington State expressed itself directly during six explosive magmatic eruptions in 1980 (18 May, 25 May, 12 June, 22 July, 7 August and 17 October 1980). This magma produced the distinctive plinian, explosive eruptions for which the volcano is famous. After three of these explosive eruptions (12 June, 7 August and 17 October), near-surface magma had low enough steam pressures so that viscous lava flows formed three consecutive, dome-shaped structures within the crater. The first two dacite lava domes built within the crater (late June and early August 1980) were destroyed by subsequent explosive eruptions (22 July and 17 October). The third dacite lava dome began to appear on 18 October 1980 above the lip of a 25-metre-diameter feeding conduit.
The new dacite lava dome
After 18 October 1980, this third and newest composite dome of dacite began to appear. By October 1986 this newest lava dome had grown within the horseshoe-shaped crater to be an immense structure up to 350 m high and up to 1,060 m in diameter (see Figures 1 and 2). The lava dome formed by a complex series of lava extrusions, supplemented occasionally by internal inflation of the dome by shallow intrusions of dacite magma into its molten core. Extrusions of lava produced short (200-400 m) and thick (20-40 m) flows piled on top of one another.2 Most dacite flows extended as lobes away from the top-centre of the dome, generally crumbling to very blocky talus on the flanks of the dome before reaching the crater floor (see Figure 3).

Between 18 October 1980 and 26 October 1986, seventeen episodes of dome growth added 74 million cubic meters of dacite to this third and newest dome.3 During these eruptions magma viscosity was high and steam pressure was low so that the magma did not express itself explosively as it had during the six earlier events of 1980. The structure produced within the crater during the six-year period was an elliptical dome of dacite lava flows and intrusions 860 m (diameter east-west), by 1,060 m (diameter north-south), by 350 m (height above northern base). During the six-year period of building of the dacite dome, there was a steady decrease with time in the volume of magma extruded. On 26 October 1986, magma movement into the dome ceased and solidification of magma began within the neck of the volcano beneath the lava dome. Eruptions after 26 October 1986 were phreatic steam explosions, not direct expressions of magma. The stability of this third dome, along with decrease in the frequency of earthquakes and phreatic steam eruptions in the ten years after October 1986, indicate that the volcano, again, may be approaching a period of dormancy.
The SiO2 content of 69 samples of the 1980 to 1986 lava dome at Mount St Helens is 63.0 ± 0.4 percent.4 Called a ‘porphyritic dacite’,5 the rock averages about 55 percent fine-grained, grey groundmass and 45 percent phenocrysts and lithic inclusions (see Figure 4). The groundmass of the rock is composed of microphenocrysts of plagioclase, orthopyroxene, and Fe-Ti oxides within a glass matrix.6 Later flows on the lava dome showed a tendency toward higher crystallinity of the groundmass7 and about 1 percent greater SiO2.8 Phenocrysts of plagioclase (30–35 percent), orthopyroxene (5 percent), hornblende (1–2 percent), Fe-Ti oxides (1 to 2 percent), and clinopyroxene (less than 0.5 percent) together comprise almost half of the lava dome.9 Lithic inclusions of gabbro, quartz diorite, hornfelsic basalt, dacite, andesite and vein quartz together compose 3.5 percent of the dome dacite.10 Of the lithic inclusions 85 percent are medium grained gabbros with an average diameter of 6 cm.11 The high mafic mineral content of gabbroic inclusions makes a small but significant decrease in the overall SiO2 content of the dacite lava dome.12

Geologists are in general agreement concerning the crustal source of the dacitic magma beneath Mount St Helens. Experimental data from the assemblage of minerals in the dacite indicate that just prior to the 18 May 1980 eruption the upper part of the magma chamber was at a temperature of 930°C and at a depth of about 7.2 km.13 That magma is believed to have contained about 4.6 weight% total volatiles, mostly H2O.14 The last dome-building intrusion event of 1986 delineated two aseismic zones (from 7–12 km and from 3–4.5 km depth) indicating that the deep magma chamber has a shallow magma-storage region.15 Fe-Ti oxide pairs indicated magmatic temperatures decreasing to about 870°C in 1986 when flows into the lava dome stopped.16
Sample collection and preparation
In June 1992, a seven-kilogram sample of dacite was collected from just above the talus apron on the farthest-north slope of the lava dome. Because the sample comes from the sloping surface of the dome, it most likely represents the upper surface of a flow lobe. The flow interpretation of the sample is corroborated by the ‘breadcrust appearance’ of dacite at the sample location, the blocky fracture pattern which suggests the toe of a lava flow, and the presence of dacite scoria just above the sample. The position on the dome suggests that the sample represents the surface of one of the last lava flows, probably from the year 1986.
| Oxide or Element | Abundance |
|---|---|
| SiO2 | 67.50% |
| Al2O3 | 16.10% |
| TiO2 | 0.61% |
| Fe2O3 | 3.97% |
| MnO | 0.06% |
| CaO | 4.18% |
| MgO | 1.27% |
| K2O | 1.69% |
| Na2O | 4.78% |
| P2O5 | 0.17% |
| Cr2O3 | < 0.01% |
| Rb | 44 ppm |
| Sr | 450 ppm |
| Y | 13 ppm |
| Zr | 190 ppm |
| Nb | 30 ppm |
| Ba | 411 ppm |
| Loss on Ignition | 0.05% |
| TOTAL | 100.5% |
| Table 1. Major-element and trace-element abundances in the 1986 dacite lava flow at Mount St Helens determined by X-ray fluorescence. The analysis was performed on dacite groundmass and phenocrysts without lithic inclusions. | |
The composition of the sample matches closely the published mineralogic, petrographic and chemical descriptions of ‘porphyritic dacite’.17 Phenocrysts of the sample are of the kind and abundance representative of the entire lava dome. The sample even has several gabbroic inclusions of the composition and size representative of the whole lava dome.18 The chemical analysis of the sample’s groundmass with phenocrysts (without gabbroic inclusions) gave 67.5 percent SiO2 by the X-ray fluorescence method (see Table 1). If the gabbroic inclusions were included in the whole rock analysis, the dacite would be about 64 percent SiO2, the average composition of the 1986 flows on the lava dome. Normative minerals were calculated in Table 2, with the assemblage representative of dacite. Thus, this seven-kilogram sample of dacite is representative of the whole lava dome.
One kilogram of dacite groundmass with phenocrysts (without gabbroic inclusions) was removed from the sample for potassium-argon analysis. The technique began by crushing and milling the dacite in an iron mortar. Particles were sieved through the 80 mesh (0.18 mm) screen and collected on top of the 200 mesh (0.075 mm) screen. The 80–200 mesh (0.18–0.075 mm) particles were specified by the argon lab to be the optimum for the argon analysis.
A second, one-kilogram sample of dacite groundmass was subsequently processed to concentrate more of the pyroxene. This separate preparation utilized crushed particles sieved through a 170 mesh (0.090 mm) screen and collected on a 270 mesh (0.053 mm) screen. These finer particles (0.053–0.090 mm) were found to allow more complete concentration of the mineral phases, even though these particles were finer than the optimum requested by the lab.
Because of the possibility of particles finer than 200 mesh absorbing or releasing a larger portion of argon, particles passing through the 200-mesh screen were rejected. The only exception was the single preparation made from particles passing through 170 mesh and collected on the 270-mesh screen.
Throughout the crushing, milling, sieving and separation processes, great care was taken to avoid contamination. The specific steps used to stop or discover contamination of the samples included:
-
Sawing of rock from the interior of the collected block of dacite (used to remove particles adhering to the sample),
-
Washing all surfaces and screens that were to contact directly the sample,
-
Final wet sieving of particles on the 200-mesh screen (or 270-mesh screen) to insure removal of finer particles (including possible contaminant lab dust introduced during milling),
-
Filtration of heavy liquids to remove contaminants,
-
Microscopic scanning of particle concentrates for foreign particles,
-
Preparation of the second concentrate from the raw dacite sample involving completely separate milling and screening (in order to discover if contamination had occurred in one of the concentrates), and
-
Sealing of samples in vials between preparation steps.
Five concentrates included one whole-rock powder and four mineral preparations. The concentrate names and descriptions are:
DOME-1 ‘Whole-rock preparation’ composed of representative particles from both the dacite groundmass and phenocrysts, without lithic inclusions; particles 80–200 mesh.
DOME-1L ‘Feldspar-glass concentrate’ from the groundmass and phenocrysts; particles 80–200 mesh; mostly plagioclase, but also contains fragments from the glassy matrix.
DOME-1M ‘Heavy-magnetic concentrate’ from the groundmass and phenocrysts; mostly hornblende with Fe-Ti oxides; particles 80–200 mesh.
DOME-1H ‘Heavy-nonmagnetic concentrate’ from the groundmass and phenocrysts; mostly orthopyroxene; particles 80–200 mesh.
DOME-1P ‘Pyroxene concentrate’ from the groundmass and phenocrysts; particles 170–270 mesh; prepared from separate dacite sample in fashion similar to DOME-1H, but with more complete concentration of orthopyroxene.
| Normative Mineral (Formula) | % by Weight |
|---|---|
| Quartz (SiO2) | 23.02 |
| Orthoclase (KAlSi3O8) | 9.95 |
| Albite (NaAISi3O8) | 40.24 |
| Anorthite (CaAI2Si2O8) | 17.40 |
| Diopside (CaMgSi2O6) | 0.94 |
| Hedenbergite (CaFeSi2O6) | 0.82 |
| Enstatite (MgSiO3) | 1.53 |
| Ferrosilite (FeSiO3) | 1.52 |
| Magnetite (Fe3O4) | 3.04 |
| Ilmenite (FeTiO3) | 1.15 |
| Apatite (Ca3P2O8) | 0.39 |
| TOTAL | 100.0 |
| Table 2. Idealized normative mineral assemblage for the Mount St Helens dacite calculated from the major-element abundances of Table 1. | |
The last four mineral concentrates were prepared from the whole rock by heavy liquid and magnetic separation. First, the representative particles from the groundmass and phenocrysts were dispersed in tribromomethane (CHBr3), a heavy liquid with a density of 2.85 g/cc at room temperature. These particles and heavy liquid were centrifuged in 250 ml bottles at 6,000 rpm. After ten minutes of centrifugation at 20°C, the float particles were collected, filtered, washed, dried and labeled. This float concentrate, ‘DOME-1L’, was more than 90 percent of the original and became the ‘feldspar-glass concentrate’. The heavy-mineral residue that sank in the heavy liquid was collected, filtered, washed and dried. It was discovered that the heavy concentrate could be separated into ‘strongly magnetic’ and ‘weakly magnetic’ fractions, with about one-third of the heavy residue being strongly magnetic. The heavy concentrate was divided by a very strong hand magnet on a large piece of filter paper at a 45° slope angle. The ‘heavy magnetic’ fraction, later labeled ‘DOME-1M’, was composed of heavy particles which climbed up the paper at 45° slope above the influence of the magnet which was moved under the paper. The residue that did not move up the filter paper was the ‘heavy-nonmagnetic’ fraction. It was labeled ‘DOME-1H’. A fourth mineral concentrate was prepared from a completely separate portion of the dacite sample and processed similar to DOME-1H except from finer particles (170–270 mesh). This finer, heavy-nonmagnetic fraction separated from the dacite was labeled ‘DOME-1P’.
Microscopic examination of the four mineral concentrates indicated the effectiveness of the separation technique. The ‘feldspar-glass concentrate’ (DOME-1L) was dominated by plagioclase and glass, with only occasional mafic microphenocrysts visible in the plagioclase and glass. Although not a complete separation of non-mafic minerals, this concentrate included plagioclase phenocrysts (andesine composition with a density of about 2.7 g/cc) and the major quantity of glass (density assumed to be about 2.4 g/cc). No attempt was made to separate plagioclase from glass, but further use of heavy liquids should be considered.
The ‘heavy-magnetic concentrate’ (DOME-1M) was dominated by amphibole minerals, with hornblende assumed to be the most abundant magnetic mineral within the dacite. However, there was also a significant amount of Fe-Ti oxide minerals, probably magnetite and ilmenite. The ‘heavy-magnetic concentrate’ also had glassy particles (more abundant than in the ‘heavy-nonmagnetic concentrate’). Mafic microphenocrysts within these glassy particles were probably dominated by the strongly magnetic Fe-Ti oxide minerals. The microscopic examination of the ‘heavy-magnetic concentrate’ also revealed a trace quantity of iron fragments, obviously the magnetic contaminant unavoidably introduced from the milling of the dacite in the iron mortar. No attempt was made to separate the hornblende from the Fe-Ti oxides, but further finer milling and use of heavy liquids should be considered.

The ‘heavy-nonmagnetic concentrate’ (DOME-1H) was dominated by orthopyroxene with much less clinopyroxene, but had a significant quantity of glassy particles attached to mafic microphenocrysts and fragments of mafic phenocrysts along incompletely fractured grain boundaries. These mafic microphenocrysts and fragments of mafic phenocrysts evidently increased the density of the attached glass particles above the critical density of 2.85 g/cc, which allowed them to sink in the heavy liquid. This sample also had recognizable hornblende, evidently not completely isolated by magnetic separation.
The ‘pyroxene concentrate’ (DOME-1P) was dominated by orthopyroxene and much less clinopyroxene. Because it was composed of finer particles (170–270 mesh), it contained far fewer mafic particles with attached glass fragments than DOME-1H. This preparation is the purest mineral concentrate. Microscopic examination of the orthopyroxene showed it to be a high-magnesium variety, explaining why it was nonmagnetic or only weakly magnetic.
The first three mineral concentrates (DOME-1L, DOME-1M, and DOME-1H) are representative of three different assemblages within the dacite. Because only the finer than 200 mesh fraction was discarded during preparation, these three concentrates should approximately sum, according to their abundance, to make the whole rock. They may not exactly sum because of differences in grind ability of the minerals and their groundmass.
K-Ar analysis
Potassium and argon were measured in the five concentrates by Geochron Laboratories of Cambridge, Massachusetts, under the direction of Richard Reesman, the K-Ar laboratory manager. These preparations were submitted to Geochron Laboratories with the statement that they came from dacite, and that the lab should expect ‘low argon’. No information was given to the lab concerning where the dacite came from or that the rock has a historically known age (ten years old at the time of the argon analysis).
The analytic data are reported in Table 3. The concentration of K (%) was measured by the flame photometry method, the reported value being the average of two readings from each concentrate. The 40K concentration (ppm) was calculated from the terrestrial isotopic abundance using the concentration of K. The concentration in ppm of 40Ar*, the supposed ‘radiogenic argon-40’, was derived from isotope dilution measurements on a mass spectrometer by correcting for the presence of atmospheric argon whose isotopic composition is known. The reported concentration of 40Ar* is the average of two values. The ratio 40Ar/Total Ar is also derived from measurements on the mass spectrometer and is the average of two values.
The ‘age’ of each concentrate is calculated by making use of what Faure19 calls the ‘general model-age equation’:
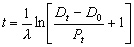
where t is the ‘age’, λ is the decay constant of the parent isotope, Dt is the number of daughter atoms in the rock presently, Do is the number of daughter atoms initially in the rock, and Pt is the number of atoms presently in the rock. Equation (1) can be used to date the rocks if measurements of Dt and Pt are made from the rock, and if an assumption concerning the original quantity of daughter (Do) is made. For the specific application to K-Ar dating,20 equation (1) becomes equivalent to equation (2) when:
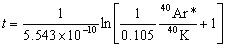
where t is the ‘age’ in millions of years, 5.543 x 10–10 yr–1 is the current estimate for the decay constant for 40K, 0.105 is the estimated fraction of 40K decays producing 40Ar, and 40Ar*/40K is the calculation by standard procedure of the mole ratio of radiogenic 40Ar to 40K in the concentrate. It should be noted that equation (1) becomes equivalent to collation (2) when
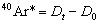
Thus, 40Ar* includes within it an assumption concerning the initial quantity of 40Ar in the rock. As a matter of practice, no radiogenic argon is supposed to have existed when the rock formed. That is, Do = 0 is supposed for equation (2) to give accurate ages. Thus, equation (2) yields a ‘model age’ assuming zero radiogenic argon in the rock when it formed. After the initial daughter assumption is made, 40Ar* is determined. Then, the mole ratio 40Ar*/40K is calculated in Table 3 from each concentrate’s 40Ar* (ppm) and 40K (ppm). Once the mole ratio is calculated (see Table 3), it is inserted into equation (2) to calculate the ‘model ages’ listed in Table 3.
| K (%) | 40K (ppm) | Total Ar (ppm) | 40Ar* (ppm) | 40Ar*/Total 40Ar | 40Ar*/40K | ‘Age’ (Ma) | |
|---|---|---|---|---|---|---|---|
|
DOME-1
‘whole rock’ |
0.924 | 1.102 | 0.0018 | 0.0000225 | 0.0125 | 0.000020 | 0.35 ± 0.05 |
|
DOME-1
feldspar, etc. |
1.048 | 1.250 | 0.0024 | 0.000025 | 0.0105 | 0.000020 | 0.34 ± 0.06 |
|
DOME-1M
amphibole, etc. |
0.581 | 0.693 | 0.0027 | 0.000037 | 0.0135 | 0.000053 | 0.9 ± 0.2 |
|
DOME-1H
pyroxene, etc. |
0.466 | 0.555 | 0.0015 | 0.000054 | 0.0360 | 0.000096 | 1.7 ± 0.3 |
|
DOME-1P
pyroxene |
0.447 | 0.533 | 0.0025 | 0.000087 | 0.0345 | 0.000163 | 2.8 ± 0.6 |
| Constants used: 40K/K = 1.193 x 10–4 g/g | Decay constant of 40K = 5.543 x 10–10 yr–1 | ||||||
| Fraction of 40K decays to 40Ar = 0.1048 | Atmospheric 40Ar/36Ar = 295.5 | ||||||
| Table 3. Potassium-argon data from the new dacite lava dome at Mount St Helens Volcano. | |||||||
Discussion
The argon analyses of the dacite lava dome show, surprisingly, a non-zero concentration of ‘radiogenic argon’ (40Ar*) in all preparations from the dacite. K-Ar ‘ages’ using equation (2) range from 0.34 ± 0.06 Ma (million years) to 2.8 ± 0.6 Ma (see Table 3). Because the sampled dacite at the time of the analyses was only ten years old, there was no time for measurable quantities of 40Ar* to accumulate within the rock due to the slow, radioactive decay of 40K. The conclusion seems inescapable that measurable 40Ar* in the dacite is not from radiogenic accumulation, but must have been resident already within the different mineral assemblages when the rock cooled from the lava in the year 1986. The lab has not measured ‘radiogenic argon’ but some other type of argon.
Other historic lava flows have been recognized to have non-zero values for 40Ar*. Of 26 historic, subaerial lava flows studied by Dalrymple,21 five gave ‘excess argon’ and, therefore, yielded excessively old K-Ar ‘ages’:
| Hualalai basalt (Hawaii, AD 1800–1801) |
1.6 ± 0.16 Ma 1.41 ± 0.08 Ma |
| Mt Etna basalt (Sicily, 122 BC) | 0.25 ± 0.08 Ma |
| Mt Etna basalt (Sicily, AD 1792) | 0.35 ± 0.14 Ma |
| Mt Lassen plagioclase (California, AD 1915) | 0.11 ± 0.3 Ma |
| Sunset Crater basalt (Arizona, AD 1064–1065) |
0.27 ± 0.09 Ma 0.25 ± 0.15 Ma |
Dalrymple22 recognized that these anomalous ‘ages’ could be caused by ‘excess radiogenic 40Ar’ from natural contamination, or caused by isotopic fractionation of argon. Krummenacher23 offered similar explanations for unexpected argon isotope ratios from several modern lava flows. Olivine, pyroxene and plagioclase from basalts of the Zuni-Bandera volcanic field (Quaternary of New Mexico) showed very significant quantities of excess argon inherited from the magmatic sources.24 The same conclusion applies to olivine and clinopyroxene phenocrysts from Quaternary volcanoes of New Zealand.25 Significant excess argon was also found in submarine basalts from two currently active Hawaiian volcanoes, Loihi Seamount and Kilauea.26
What caused the non-zero 40Ar* in the Mount St Helens dacite? Could contaminant 40Ar in the laboratory have been added to the Mount St Helens dacite giving the impression of great age? The possibility of contamination caused extreme care to be taken in cleaning the processing equipment, and the concentrates were sealed tightly in vials between preparation and analysis. Could the processing equipment itself be adding argon? For example, might the iron fragments produced during milling the sample in the mortar add argon? The heavy-liquid separation process strongly rejects heavy iron from the light feldspar-rich assemblage (preparation DOME-1L), but this concentrate also contains significant 40Ar. Other processes seem to exclude or isolate laboratory contamination. The wet sieving on the 200-mesh screen, for example, should remove any fine lab dust which could have fallen onto the concentrates. Because of these extraordinary considerations, laboratory contamination of the five concentrates is a very remote possibility.
Could the magmatic process beneath the lava dome be adding a contaminant to the molten dacite as it ascends from great depth? This is a possibility needing consideration. Might an argon-rich mineral (‘xenocryst’) be added to the magma and impart an excessive age to the ‘whole rock’ dacite? The data of Table 3 seem to argue that very different mineral phases of the dacite each contain significant 40Ar. Although the mineral concentrates are not pure, and all contain some glass, an argument can be made that both mafic and non-mafic minerals of the dacite contain significant 40Ar. The lithic inclusions in the lava dome might be thought to be the contaminant, in which case they might add ‘old’ mafic and non-mafic minerals to the young magma. It could be argued that gabbroic clumps in the magma disaggregated as the fluidity of the magma decreased with time, thereby adding an assortment of ‘old’ mineral grains. However, Heliker27 argues that the gabbroic inclusions are not xenoliths from the aged country rock adjacent to the pluton, but cumulates formed by crystal segregation within a compositionally layered pluton. These inclusions are, therefore, regarded as a unique association within the recent magmatic system.
Could the magmatic conditions at depth allow argon to be occluded within the minerals at the time of their formation? This last, and most interesting, explanation of the anomalous 40Ar suggests the different quantities of argon in different mineral assemblages are caused by variation in the partial pressure of the gas as crystallization progressed, or by different quantities of gas retained as pressure was released. Crystallization experiments by Karpinskaya28 show that muscovite retains up to 0.5 percent by weight argon at 640°C and vapour pressure of 4,000 atmospheres. Phenocryst studies by Poths, Healey and Laughlin29 showed that olivine and clinopyroxene separated from young basalts from New Mexico and Nevada have ‘ubiquitous excess argon’. A magmatic source was postulated for the argon in phenocrysts of olivine and clinopyroxene in Quaternary volcanics of New Zealand.30 Presumably other minerals occlude argon in relation to the partial pressure of the gas in the magma source.
Laboratory experiments have been conducted on the solubility of argon in synthetic basaltic melts and their associated minerals.31, 32 Minerals and melts were held near 1300°C at one atmosphere pressure in a gas stream containing argon. After the material was quenched, the researchers measured up to 0.34 ppm 40Ar within synthetic olivine. They noted, ‘The solubility of Ar in the minerals is surprisingly high’.33 Their conclusion is that argon is held primarily in lattice vacancy defects within the minerals.
Argon occlusion within mineral assemblages is supported by the data from the dacite at Mount St Helens. Table 3 indicates that although the mineral concentrates (rich in feldspar, amphibole or pyroxene) have about the same ‘Total Ar’ concentrations, the ‘pyroxene concentrate’ possesses the highest concentration of 40Ar* (over three times that of the ‘feldspar-glass concentrate’) and the highest proportion of 40Ar* (40Ar*/Total Ar is over three times that of the ‘feldspar-glass concentrate’). These data suggest that whereas the orthopyroxene mineral structure has about the same or slightly less gas retention sites as does the associated plagioclase, orthopyroxene has a tighter structure and is able to retain more of the magmatic40Ar. Orthopyroxene retains the most argon, followed by hornblende, and finally, plagioclase. According to this interpretation, the concentration of 40Ar* of a mineral assemblage is a measure of its argon occlusion and retention characteristics. Therefore, the 2.8 Ma ‘age’ of the ‘pyroxene concentrate’ has nothing to do with the time of crystallization.
Where does the argon in the magma come from? Could it be from outgassing of the lower crust and upper mantle? More study is needed.
To test further the hypothesis of argon occlusion in mineral assemblages, higher purity mineral concentrates could be prepared from the dacite at Mount St Helens. Finer-grained concentrates should be processed more completely with heavy liquids and magnetic separation. The preparation of DOME-1P, a finer-grained and purer pyroxene concentrate than DOME-1H, has, as expected, a higher concentration of 40Ar* and lower concentration of 40K. Acid-solution techniques or further use of heavy liquids could also help to remove undesirable glass. The glass itself should be concentrated for analysis of argon.
Applications to other K-Ar ages
Do other volcanic rocks with phenocrysts have mineral assemblages with generally occluded argon? Phenocrysts are very common in volcanic rocks, so a general test of the hypothesis could be devised. In addition to testing other historic lava flows, phenocrysts from some ancient flows might be tested for phenocrysts which greatly exceed the ‘whole rock’ age. Three possible applications are suggested here.
-
Basalt of Devils Postpile (Devils Postpile National Monument, California)
Plagioclase separated from the Devils Postpile basalt gave a K-Ar ‘age’ of 0.94 ± 0.16 million years.34 The basalt has been reassigned recently an age of less than 100,000 years based on new geologic mapping and detailed stratigraphic study.35 What was the cause of the excessively old age? It could be argon occluded within the plagioclase.
-
Basalt of Toroweap Dam (western Grand Canyon, Arizona)
The basalt of Toroweap Dam lies at the bottom of Grand Canyon very near the present channel of the Colorado River. The basalt has been dated twice by the K-Ar method at 1.16 ± 0.18 Ma and 1.25 ± 0.2 Ma.36 The original researchers qualified their statements concerning the basalt date by saying, ‘There is the possibility that pre-eruption argon was retained in the basalt’.37 Many other basalts of western Grand Canyon have been shown to contain ‘excess argon’.38 Although the original researchers do not express certainty concerning the K-Ar age of the basalt at Toroweap Dam, other geologists have assigned much greater certainty and use the K-Ar age to argue that Grand Canyon has existed for a very long time (see especially D.A. Young39).
-
Keramim basalt (northern Golan Heights, Israel)
‘Stone Age’ artifacts occur beneath Keramim basalt dated at 0.25 Ma by the K-Ar method.40 However, human occupation is not thought to have occurred in Israel during the Lower Palaeolithic,40 so this and other K-Ar ‘ages’ should be checked. Because the K-Ar method has been used elsewhere to date Neanderthal Man, we might ask if other Neanderthal ‘ages’ need careful scrutiny.
Conclusion
Argon analyses of the new dacite lava dome at Mount St Helens raise more questions than answers. The primary assumption upon which K-Ar model-age dating is based assumes zero 40Ar* in the mineral phases of a rock when it solidifies. This assumption has been shown to be faulty. Argon occlusion in mineral phases of dacite at Mount St Helens is a reasonable alternate assumption. This study raises more fundamental questions—do other phenocryst-containing volcanic rocks give reliable K-Ar ages?
Acknowledgments
Financial support was provided by the Institute for Creation Research and Mr Guy Berthault. Dr Andrew Snelling provided helpful comments and reviews of the manuscript.
References and notes
- Pringle, P.T., Roadside Geology of Mount St Helens National Volcanic Monument and Vicinity, Washington State Department of Natural Resources, Washington Division of Geology and Earth Resources, Information Circular 88, p. 120, 1993.
- Swanson, D.A. and Holcomb, R.T., Regularities in growth of the Mount St Helens dacite dome, 1980–1986. In: Lava Flows and Domes, J. Fink (ed.), Springer-Verlag, Heidelberg, Vol. 2, pp. 3–24, 1990.
- Swanson and Holcomb, Ref. 2.
- Swanson and Holcomb, Ref. 2.
- Cashman, K.V., Crystallization of Mount St Helens 1980–1986 dacite: a quantitative textural approach, Bulletin Volcanologique 50:194–209, 1988.
- Cashman, K.V. and Taggart, J.E., Petrologic monitoring of 1981 and 1982 eruptive products from Mount St Helens, Science 221:1385–1387, 1983.
- Cashman, K.V., Groundmass crystallization of Mount St Helens dacite, 1980–1986: a tool for interpreting shallow magmatic processes, Contributions to Mineralogy and Petrology 109:431–449, 1992.
- Swanson and Holcomb, Ref. 2.
- Cashman, Ref. 5
- Heliker, C., Inclusions in Mount St Helens dacite erupted from 1980 through 1983, Journal of Volcanology and Geothermal Research 66:115–135, 1995.
- Heliker, Ref. 10.
- Heliker, Ref. 10.
- Rutherford, M.J., Sigurdsson, H., Carey, S. and Davis, A., The May 18, 1980 eruption of Mount St Helens 1: melt composition and experimental phase equilibria, Journal of Geophysical Research 90:2929–2947, 1985.
- Rutherford, M.J. and Devine, J.D., The May 18, 1980 eruption of Mount St Helens 3: stability and chemistry of amphibole in the magma chamber, Journal of Geophysical Research 93:11949–11959, 1988.
- Endo, E.T., Dzurisin, D. and Swanson, D.A., Geophysical and observational constraints for ascent rates of dacitic magma at Mount St Helens. In: Magma Transport and Storage, M.P. Ryan (ed.), John Wiley and Sons, New York, pp. 318–334, 1990.
- Cashman, Ref. 7.
- Cashman, Ref. 5.
- Heliker, Ref. 10.
- Faure, G., Principles of Isotope Geology, 2nd edition, John Wiley and Sons, New York, p. 42, 1986.
- Dalrymple, G.B.and Lanphere, M.A., Potassium-Argon Dating: Principles, Techniques and Applications to Geochronology, W. H. Freeman, San Francisco, p. 49, 1969.
- Dalrymple, G.B., 40Ar/36Ar analyses of historic lava flows, Earth and Planetary Science Letters, 6:47–55, 1969.
- Dalrymple, Ref. 21.
- Krummenacher, D., Isotopic composition of argon in modern surface volcanic rocks, Earth and Planetary Science Letters 8:109–117, 1970.
- Laughlin, A.W., Poths, J., Healey, H.A., Reneau, S. and Wolde Gabriel, G., Dating of Quaternary basalts using the cosmogonic 3He and 14C methods with implications for excess 40Ar, Geology 22:135–138, 1994.
- Patterson, D.B., Honda, M. and McDougall, I., Noble gases in mafic phenocrysts and xenoliths from New Zealand, Geochimica et Cosmochimica Acta 58:4411–4427, 1994.
- Honda, M., McDougall, I., Patterson, D.B., Doulgens, A. and Clague, D.A., Noble gases in submarine pillow basalt glasses from Loihi and Kilauea, Hawaii: a solar component in the Earth, Geochimica et Cosmochimica Acta 57:859–874, 1993.
- Heliker, Ref. 10.
- Karpinskaya, T.B., Synthesis of argon muscovite, International Geology Review 9:1493–1495, 1967.
- Poths, J., Healey, H. and Laughlin, A.W., Ubiquitous excess argon in very young basalts, Geological Society of America Abstracts with Programs 25:A–462, 1993.
- Patterson et al., Ref. 25.
- Broadhurst, C.L., Drake, M.J., Hagee, B.E. and Benatowicz, T.J., Solubility and partitioning of Ar in anorthite, diopside, forsterite, spinel, and synthetic basaltic liquids, Geochimica et Cosmochimica Acta 54:299–309, 1990.
- Broadhurst, C.L., Drake, M.J., Hagee, B.E. and Benatowicz, T.J., Solubility and partitioning of Ne, Ar, Kr, and Xe in minerals and synthetic basaltic melts, Geochimica et Cosmochimica Acta 56:709–723, 1992.
- Broadhurst et al., Ref 31.
- Dalrymple, G.B., Potassium-argon dates of three Pleistocene interglacial basalt flows from the Sierra Nevada, California, Geological Society of America Bulletin 75:753–758, 1964.
- Huber, N.K. end Eckhardt, W.W., Devils Postpile Story, Sequoia Natural History Association, Three Rivers, California, p. 30, 1985.
- Hamblin, W.K., Late Cenozoic Lava Dams in the Western Grand Canyon, Geological Society of America, Memoir 183, Boulder, Colorado, p. 139, 1994.
- McKee, E.D., Hamblin, W.K. and Damon, P.E., K-Ar age of lava dam in Grand Canyon, Geological Society of America Bulletin 79:133–136, 1968.
- Hamblin, Ref. 36.
- Young, D.A., The discovery of terrestrial history. In: Portraits of Creation: Biblical and Scientific Perspectives on the World’s Formation, H.I. Van Till, R.E. Snow, J.H. Stek and D.A. Young (eds), William B. Eerdmans, Grand Rapids, Michigan, pp. 26–81, 1990.
- Mor, D., Har Odem Geological Map, Geological Survey of Israel, Jerusalem, scale 1:50,000, one sheet, 1987.
- Bar-Yosef, O., Geochronology of the Levantine Middle Palaeolithic. In: The Human Revolution, P. Mellars and C. Stringer (eds), Princeton University Press, Princeton, New Jersey, pp. 589–610, 1989.




Readers’ comments
Comments are automatically closed 14 days after publication.