Journal of Creation 29(1):80–87, April 2015
Browse our latest digital issue Subscribe
Phytogeography and zoogeography—rafting vs continental drift
Evolutionists have great difficulties explaining the global distributions of plants and animals. Accepted models of continental drift are inadequate to explain both trans-Atlantic and trans-Pacific disjunctions. At the same time, evolutionary biogeographers are unable to provide an adequate mechanism by which these distribution patterns could have arisen by dispersal. In contrast, the data fit well within a creationist model where plants and animals were rafted to the places they now inhabit on log mats left over from the Genesis Flood. The more raftable animals tend to have the most numerous transoceanic disjunctions and areas of high endemism/biodiversity tend to be concentrated in coastal regions where ocean currents intersect with land masses. Areas of high plant endemism/biodiversity often coincide with areas of high animal endemism/biodiversity, suggesting that the plants and animals were transported to these places by the same means.

“The pattern of geographical distribution [of plants and animals] is just what you would expect if evolution had happened.” (Richard Dawkins, Oxford University 1)
“Biogeography (or geographical distribution of organisms) has not been shown to be evidence for or against [macro] evolution in any sense.” (Gareth Nelson and Norman Platnick, American Museum of Natural History 2)
Disjunct distributions, where similar plants and animals are found in widely separated areas, are numerous. Moreover, many patterns of disjunction are seen, giving rise to the concept of ‘tracks of dispersal’ (figure 1). In the preface to their Cladistic Biogeography, Humphries and Parenti argue that “These ‘generalised tracks’ of distribution are so consistent in disjunct, transoceanic terrestrial taxa … that they imply historical connections between the biotas.”3 Many of these ‘historical connections’, it is argued, can be explained by continental drift and the associated fragmentation of widespread ancestral species.
Among geologists, the generally accepted model has the supercontinent Gondwana rifting to form the Atlantic Ocean, with Africa splitting off from South America about 120 Ma ago. This, however, is poorly supported by biogeographic data. Of about 200 seed plant families native to eastern South America, only 156 are common to eastern South America and West Africa, whereas 174 are common to eastern South America and eastern Asia.5 This hardly fits the view that, prior to the rifting of Gondwana, South America and Africa were joined for millions of years. Moreover, hundreds of plants found in both South America and Africa are classified as being the same species. How, then, can they have been separated for over a hundred million years? Given the alleged power of evolution, it would seem remarkable that they haven’t changed significantly over such a long period of time. Furthermore, according to evolution theory, many plants and animals with transoceanic disjunct distributions originated millions of years after the continents are said to have drifted apart.6-11

In order to explain the hundreds of trans-Pacific disjunctions, some biogeographers have rejected the geologists’ model of Gondwana rifting to form the Atlantic Ocean, in favour of an alternative supercontinent, Pacifica, rifting to form the Pacific Ocean.12 Another scenario proposed to explain transoceanic disjunctions is the ‘Expanding Earth’ hypothesis. This postulates that, prior to the Jurassic period, the Earth was smaller with all its present oceans closed and that an increase in Earth’s radius gave rise to both the Pacific and Atlantic Oceans.13-14
Further confusion about Earth’s geological history arises from the many anti-tropical distributions where plants and animals are disjunct across the tropics, i.e. they are found in the northern and southern regions but not in between.15 This has led some evolutionists to postulate a pre-Pangaean configuration whereby the present northern and southern regions were once adjacent to one another.16 As admitted by Van Damme and Sinev: “None of the theories can reconcile the current geological and biogeographical data.”17
The difficulties evolutionists have in explaining biogeographic patterns have led to the most remarkable admissions. Nelson and Platnick wrote of how biogeography lends itself “to ever more complicated treatment in the abstract, which is apt to border even on the miraculous, but which is apt to crumble in confrontation with concrete facts of life”. Similarly, Croizat opined:
“… contemporary zoogeographers founder in a self-created morass of chance hops; great capacities for, or mysterious means of, dispersal; rare accidents of over-sea transportation; small probabilities that with time become certainties; and other pseudoexplanations.”18
The 1998 biogeography symposium of the Willi Hennig Society argued that “historical biogeography was in a mess, a subject looking for a method”.19 Writing prior to the general acceptance of plate tectonics, Darlington commented: “I have tried … to see if I can find any real signs of [continental] drift in the present distribution of animals. I can find none.”20
The logical alternative—dispersal
Despite their inability to correlate biogeographic data with their beliefs about Earth history, evolutionists often reject the alternative of dispersal across oceans. This is because it “is thought to be a random process, and hence it could not have given rise to the type of congruent or concordant patterns found in so many different groups.”21 Had these biogeographers believed what the Bible teaches about Earth history, however, they might have been more open-minded. The Genesis Flood would have uprooted billions of trees, many of which would have been left floating upon the oceans. These massive islands of vegetation could have easily dispersed both plants and animals around and across oceans, especially given the high levels of rainfall arising from the warm post-Flood oceans.22 Moreover, their being propelled by ocean currents would explain the consistency of the many clear patterns of disjunction (figure 1) and the general correspondence between areas of high biodiversity and the intersection of ocean currents with land masses (figure 15).23 Ironically, in discussing Croizat’s tracks of dispersal, Humphries and Parenti remark:
“Characteristically, many disjunct patterns spanned ocean bottoms, to the point that the oceans have been characterized as the natural biogeographic regions and the continents represent the land areas around the periphery.”24

Rafting of animals?
In discussing the plausibility of reptiles and mammals traversing significant stretches of water, it should be remembered that the safe arrival of just one pregnant female would be sufficient to establish a new colony. Moreover, there are numerous examples of sizeable islands of vegetation being seen adrift at sea.25-26 Charles Lyell reported that rafts had been seen floating on the Amazon carrying snakes, alligators, monkeys, and squirrels and that, on one occasion, four pumas had rafted down the Parana River to Montevideo where they were discovered prowling the streets!27 Alfred Wallace records that a large boa constrictor floated 320 km (200 miles) from the island of Trinidad to the island of St Vincent, wrapped around the trunk of a cedar tree.28 Another account involved a pirate who, having been marooned on a riverbank in hostile territory, swam to a floating nipa palm island and remained adrift for several days subsisting on the palm fruits.29 Following the Indian Ocean tsunami of 2004, a man survived being swept out to sea for eight days, clinging to a floating tree and drinking rainwater. He was picked by a passing ship 160 km (100 miles) offshore.30 One raft was spotted in the Atlantic, intact with trees 9 m (30 feet) high, despite having rafted along the coast of North America for over 1,600 km (1,000 miles).31 Schuchert records how one such raft was seen carrying live lizards, snakes, and small mammals as far as 1,600 km (1,000 miles) out to sea.32 Moreover, rafts left over from the Genesis Flood would surely have dwarfed such as these.

Woodmorappe33 has documented how rough waters tend to concentrate rather than disperse natural rafts, with vegetation debris tending to be rolled into tight clumps. He also discusses another major source of flotsam, i.e. pumice. This is known to cover large areas—with a thickness sufficient for a man to walk on34—and can float in the ocean for years. The considerable volcanic activity occurring during the Flood may have produced islands of pumice thousands of square metres in area.
Zoogeography provides an opportunity to test the hypothesis that rafting played a significant role in dispersing plants and animals to their present habitats. Particularly, we would expect to see a correlation between raftability and frequency of transoceanic disjunction, with more raftable animals having a higher incidence of disjunction.

Small animals
Samples of flotsam have been found to be remarkably rich in insects and other small animals including snails, spiders, mites, millipedes, isopods, worms, and pseudoscorpions.35-36 Numerous insect disjunctions are known across both the Pacific and Atlantic Oceans.37-39 Although it might be argued that flying species could have been dispersed by wind, examples are also known in flightless insects such as the cricket subfamily Macropathinae40 and the flea subfamily Stephanocircinae.41 In addition, many transoceanic disjunctions are known among arachnids, (e.g. Micropholcommatidae (figure 2), Pettalidae (figure 3), Neogoveidae, Mecysmaucheniidae, Palpimanidae, Archaeidae, Chthoniidae, Tridenchthoniidae, Garypidae, Zalmoxidae and Olpiidae), millipedes (e.g. Heterochordeumatoidea, Spirostreptidae, Iulomorphidae, Cambalidae, Spirobolellidae, Rhinocricidae, Stemmiulidae, Siphoniulidae, Siphonotidae, Pygrodesmidae, Platyrhacidae, Fuhrmannodesmidae and Cryptodesmidae),42 planarians, earthworms and snails.17

Reptiles
Reptiles are among the most raftable animals due to their tolerance of salt water and their ability to subsist for long periods without eating or drinking.45 The ability of reptiles to traverse wide stretches of ocean is illustrated by the presence of Scincidae on many islands in the western Pacific and by the gecko species, Nactus pelagicus and Gehyra vorax, both of which are distributed throughout many islands in the southern Pacific.46

Reptiles, generally, are widely distributed with many transoceanic disjunctions. Examples include side-necked turtles of the family Chelidae (figure 4), turtles of the family Geoemydidae (figure 5), skinks of the family Scincidae (figure 6), lizards of the family Ignuanidae (figure 7), turtles of the family Podocnemididae (figure 8), and boine snakes of the family Colubridae (figure 9). Other transoceanic reptile disjunctions include Pelomedusidae, Geoemydidae, Emydidae, Testudinidae, Crocodylinae, Amphisbaenidae, Aniliidae, Anguidae, Alligatoridae, Dibamidae, Mabuyinae, Testudinidae, Typhlopidae, Trionychidae, Leptotyphlopidae, Crotalinae, and Gekkonidae. The lizard genus Sphenodon is found in New Zealand and the gecko Tarentola made its way 6,000 km from North Africa to Cuba.47
Amphibians
Amphibians are less raftable than reptiles due to their lower tolerance of salt water. However there is little doubt that rafting of some amphibian groups is possible due to the presence of frogs on a significant number of oceanic islands. (It would seem that rafting provides the only viable explanation for their distribution).56 There are fewer transoceanic disjunctions involving amphibians than reptiles but there are some, including Microhylinae (figure 10), Pipidae (figure 11), Leptodactylidae and Leiopelmatidae, all of which are anurans.57 The pelodryadine hylids of Australia and New Guinea are closely related to a group of hyloids that exists only in South America. Even evolutionists have concluded that these must have crossed the Pacific as, in their thinking, pelodryadine hylids evolved tens of millions of years after the alleged Antarctic land bridge between South America and Australia disappeared.58
There are fewer transoceanic disjunctions involving caecilians (figure 12) and they are conspicuous by their absence in Australasia. Arguably, there are none involving urodeles (figure 13) as these may have dispersed across a warm Bering land bridge.59-60 Giant salamanders (Crytobranchdae), for example, are found in eastern Asia and eastern North America and may have been part of a continuous plant and animal distribution linking these two regions.61 Whereas there are numerous reptiles found on Madagascar (geckos, chameleons, skinks, iguanids, snakes, turtles and tortoises, and crocodiles) the amphibians comprise just four frog families.

The ability of relatively salt-intolerant amphibians to raft significant distances can be explained by the likely size of the post-Flood vegetation mats and high levels of post-Flood rainfall.22 There are a number of possible explanations for the greater raftability of anurans compared with urodeles. Anuran larvae are usually omnivorous and many will eat the eggs of their own species; urodele larvae are only carnivorous.62 Also, fewer urodeles exhibit salt tolerance than anurans.63 One anuran species, Fejervarya cancrivora, is particularly salt tolerant and is known to live in brackish environments such as mangrove swamps. It also swims happily in full-strength sea water.64 Platymantine frogs, which are widely distributed from the Philippines to Fiji, lay terrestrial eggs and forego the tadpole stage, giving them a greater chance of rafting across salt water. Arboreal anurans do not require large amounts of water and could have sheltered in upright trees, well away from sea water. The fossorial nature of caecilians would make them less likely to be found on rafts than anurans.

Mammals
Mammals are among the least raftable of terrestrial animals as they require substantial amounts of water to survive even for short periods. However, some can obtain the water they need from vegetation. Rodents are a well-known example of this and are found on many isolated oceanic islands.71 The few transoceanic mammal disjunctions include monkeys,72 rodents,73 the bat families Molossidae and Emballonuridae (all of which are disjunct across the Atlantic) and the bat superfamily Noctilionoidea which is disjunct across the Pacific.74 Another interesting example is the marsupial Dromiciops found in Chile but which is more closely related to Australian marsupials than other South American marsupials.75 The other South American marsupials probably arrived by migration from Asia across a Bering land bridge.23,59
More evidence of rafting from the Madagascan fauna

Evolutionists’ models of continental drift show Madagascar becoming separated from the mainland in the Late Cretaceous. However, its Cretaceous vertebrate fossil record bears little resemblance to its living species. According to Angelica Crottini et al.:
“The Cretaceous fauna included lungfishes, gars, nonranoid giant frogs, dinosaurs and marsupial and gondwanatherian mammals whereas the extant vertebrate fauna is composed of mainly percomorph freshwater fishes, ranoid frogs, modern squamate reptiles, lemurs, rodents, carnivores, afrotherian mammals, bats, and numerous families of birds.”76

Prior to the breakup of Gondwana, Madagascar was supposedly sandwiched between India and Africa, allowing faunal interchange between South America, Africa, Madagascar and India (figure 14). This is said to explain why the lizard family Iguanidae (figure 7), the turtle family Podocnemidae (figure 8) and the boa subfamily Boinae (figure 9) are found in both South America and Madagascar. However, there are no living representatives of these groups in either Africa or India. Moreover, evolutionists’ DNA analyses are said to show that their ancestors became separated about 80 Ma ago; but this is over 20 Ma after continental drift allegedly broke the land connection between Madagascar and South America. In an attempt to solve this problem, some have suggested that land bridges existed between Madagascar and Antarctica up until the Late Cretaceous, enabling faunal interchange between Madagascar and South America via Antarctica.77 Others, however, are adamant that such land bridges did not exist!78-79 Similarly, Madagascan net-winged midges of the subfamily Edwardsininiae are absent in both Africa and India, but are found in South America and Australia.
Madagascar supposedly remained in contact with India for 50 Ma after it was separated from Africa but the Madagascan termite fauna is more closely related to that of Africa than India.80 Darlington observes:
“The Madagascan fauna … seems to be an accumulation of animals received from two directions [Africa and the Orient] … rather than part of a fauna exchanged by Africa and India across Madagascar.”81

Unsurprisingly, some evolutionists concede that much of Madagascar’s extant terrestrial fauna probably colonised the island by rafting.82-83
Areas of endemism/high biodiversity
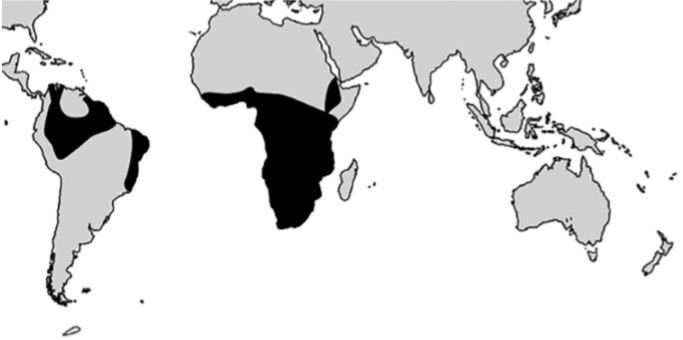
Plants and animals are not distributed randomly. Instead they tend to be clustered in ‘areas of high endemism’ (regions where there are a high number of endemic species) or ‘areas of high biodiversity’ (regions where there are many different species but which may not necessarily be endemic). Significantly, areas of high endemism tend to coincide with areas of high biodiversity and these are often coastal areas or islands where land masses intersect with ocean currents (figure 15).

Generally, areas of high plant endemism correspond to areas of high animal endemism.84 For example, the Yucatan peninsula of Central America is an area of endemism for amphibians, reptiles and birds. It is also one of Croizat’s nodes of plant dispersal (figure 1). Madagascar is an area of endemism for reptiles, birds and mammals. This is also another of Croizat’s nodes. The southern tip of South Africa is an area of endemism for amphibians, mammals and birds. It is also a point on Croizat’s southern track. Another area of high plant endemism is southern West Africa (Ghana and the Ivory Coast). This is also an area of endemism for amphibians, birds and mammals.85 As with areas of endemism generally, all these are regions where ocean currents might deposit rafts (figure 15).

Evolutionists, of course, will argue that high endemism would arise on islands and in coastal areas where there are many different ecological niches and therefore ideal conditions for evolutionary diversification. Moreover, these tend to be associated with areas of high rainfall, which might be argued to provide an environment where plants can thrive and many different plants and animals could evolve. However, this does not explain the many patterns of disjunction where the same plants and animals are found in the same widely separated areas of endemism.86 This is better explained by rafting and the creationists’ model of rapid post-Flood speciation.87-88 Rafting on ocean currents would transport the same plants and animals to the same widely separated regions. These would then diversify into many different species as they adapted to their new environments, giving rise to areas of high endemism. Hence, the creation model would also predict that coastal regions and islands such as Madagascar would have many endemic species.

Conclusion
Evolutionists often present an allegedly unifying theory of Earth history, claiming that the distributions of plants and animals fit well with the geologists’ model of slow continental drift and the rifting of Gondwana to form the Atlantic Ocean. The reality, however, is that the distributions of many plants and animals fit very poorly within this framework. At the same time, they support the rafting model well. There appears to be a clear association between raftability and frequency of transoceanic disjunction. Areas of high biodiversity/high endemism, generally, are found where ocean currents intersect with land masses and, most significantly, these areas often overlap for plants and animals, supporting the view that they were transported to these places by the same means. The creationist rafting model appears much superior to the evolutionist model of continental drift.
Rafting is just one of a number of means by which plants and animals could have dispersed following the Genesis Flood, other possibilities being land bridges that have subsequently fallen below sea level and transport by man.89-90 Catastrophic plate tectonics, however, is not considered as the movement of the continents would have taken place beneath the Flood waters. The study of biogeography together with the rafting model—if deemed valid—may have implications for the ongoing debate regarding the placement of the Flood/ post-Flood boundary.
References
- Global Atheist Convention, Melbourne, Australia, 2010. Return to text
- Nelson, G. and Platnick, N., Systematics and Biogeography: Cladistics and Vicariance, Columbia University Press, New York, p. 223, 1981. Return to text
- Humphries, C.J. and Parenti, L.R., Cladistic Biogeography, 2nd edn, Oxford University Press, 1999. Return to text
- Croizat, L., Panbiogeography, vols 1, 2A and 2B, self-published, 1958. Return to text
- Thorne, R.F., Floristic Relationships Between Tropical Africa and Tropical America, Smithsonian Press, 1973. Return to text
- Davis, C.C., Laurasian migration explains Gondwanan disjunctions: Evidence from Malpighiaceae, PNAS 99(10):6833–6837. Return to text
- De Queiroz, A., The Monkey’s Voyage, Basic Books, New York, p. 295, 2014. Return to text
- De Queiroz, A., The resurrection of oceanic dispersal in historical biogeography, Trends in Ecology and Evolution 20(2): 68–73, February 2005 |doi: 10.1016/j.tree.2004.11.006 Return to text
- Vidal, N. et al., Origin of tropical American burrowing reptiles by transatlantic rafting, Biology Letters 4(1):115–118, 23 February 2008 |doi: 10.1098/ rsbl.2007.0531 Return to text
- Vidal, N. et al., Blindsnake evolutionary tree reveals long history on Gondwana, Biology Letters rsbl20100220, 31 March 2010 | doi: 10.1098/rsbl.2010.0220 Return to text
- George, W. and Lavocat, R. (Eds), The Africa-South America Connection, Clarendon Press, Oxford, 1993. Return to text
- Humphries and Parenti, ref. 3, pp. 133, 134 and 151. Return to text
- Hurrell, S., Dinosaurs and the Expanding Earth, 3rd edn, One off Publishing, p. 111, 2011. Return to text
- Humphries and Parenti, ref. 3, pp. 128, 131–134. Return to text
- Humphries and Parenti, ref. 3, p. 126–128. Return to text
- Humphries and Parenti, ref. 3, pp. 150–151. Return to text
- Van Damme, K. and Sinev, A.Y., Tropical amphi-Pacific disjunction in the Cladocera (Crustacae: Branchiopoda), J. Limnology 72(s2):210, 2013. Return to text
- Sparks, J.S. and Smith, W.L., Freshwater fishes, dispersal ability and non-evidence: “Gondwana life rafts” to the rescue, Systematic Biology 54(1):158–165, 2005 | doi: 10.1080/10635150590906019 Return to text
- Humphries, C.J., Form, space and time; Which comes first? J. Biogeography 27(1):11–15, January 2000. Return to text
- Darlington, P.J., Zoogeography: The Geographical Distribution of Animals, John Wiley & Sons, New York, p. 606, 1957. Return to text
- Sanmartin, I. and Ronquist, F., Southern Hemisphere Biogeography Inferred by Event-Based Models: Plant versus Animal Patterns, Systematic Biology 53(2):216–243, 2004. Return to text
- Batten, D. et al., The Creation Answers Book, Creation Book Publishers, 3rd edn, p. 205–207, 2009. Return to text
- Statham, D.R., Migration after the Flood. How did plants and animals spread around the world so quickly?. Return to text
- Humphries and Parenti, ref. 3, p. 36. Return to text
- Metcalfe, I. et al., Faunal and Floral Migration and Evolution in SE Asia-Australasia, CRC Press, Boca Raton, FL., p. 409–414, 2001. Return to text
- Van Duzer, C., Floating Islands: A Global Bibliography, Cantor Press, Los Altos Hills, CA, pp. 362–363, 2004. Return to text
- Lyell, C., Principles of Geology, vol. III, John Murray, London, 6th edn, pp. 125–128, 1840. Return to text
- Wallace, A.R., Island Life, 2nd and revised edn, Macmillan, London, p. 75, 1895. Return to text
- Brandon-Jones, D., Pre-Glacial Bornean primate impoverishment and Wallace’s Line, in Hall, R. and Holloway, J.D. (Eds), Biogeography and Geological Evolution of SE Asia, Leiden, pp. 393–403, 1998. Return to text
- De Queiroz, ref. 7, p. 131. Return to text
- Powers, S., Floating Islands, Popular Science, September 1911, pp. 303–307; popsci.com. Return to text
- Schuchert, C., Historical Geology of the Antillean-Caribbean Region, John Wiley & Sons, New York, p. 80, 1935. Return to text
- Woodmorappe, J., Noah’s Ark: A Feasibility Study, Institute for Creation Research, Santee, CA, p. 155, 1996. Return to text
- Van Duzer, ref. 26, pp. 59–60, 366. Return to text
- Heatwole, H. and Levins, R., Biogeography of the Puerto Rican bank: flotsam transport of terrestrial animals, Ecology 53:112–117, 1972 | doi: doi. org/10.2307/1935715. Return to text
- Gillespie, R.G. et al., Long-distance dispersal: a framework for hypothesis testing, Trends in Ecology and Evolution 27(1):47–56, January 2012. Return to text
- E.g. Chironomidae, Micropholcommatinae, Ameletopsidae, Coloburiscidae, Nesameletidae, Oniscigastriade, Eustheniidae, Gripopterygidae, Austroperlidae, the water flea genus Leydigiopsis, fly genera Bactrocera, Platyroptilon, and Setostylus, 30 genera and subgenera of crane flies shared between South America and Australasia, and many others, including beetles, moths, butterflies, and dragonflies.17 Return to text
- Van Damme and Sinev, ref. 17, pp. 209–244. Return to text
- Noonan, G.R., Faunal relationships between eastern North America and Europe as shown by insects, Memoirs of the Entomological Society of Canada 20(S144):39–53, January 1988 | doi: dx.doi.org/10.4039/entm120144039-1. Return to text
- Mesa, A. et al., The karyotype of some Australian species of Macropathinae (Gryllacridoidea—Rhaphidophoridae), Chromosoma 24(4):456–466, 1968. Return to text
- Grimaldi, D. and Engel, M.S., Evolution of the Insects, Cambridge University Press, Cambridge, UK, p. 485, 2005. Return to text
- Biogeography of Millipede Families, The Field Museum, Chicago; www. fieldmuseum.org/sites/default/files/Identification_Table_3.pdf. Return to text
- Rix, M.G. and Harvey, M.S., The spider family Micropholcommatidae (Arachnida, Araneae, Araneoidea): a relimitation and revision at the generic level, ZooKeys 36:1–321, 2010 | doi: 10.3897/zookeys.36.306 Return to text
- Boyer, S.L., Biogeography of the world: a case study from cyphoph Opiliones, a globally distributed group of arachnids, J. Biogeography, 2007 | doi:10.1111/j.1365-2699.2007.01755.x. Return to text
- Thiel, M. and Gutow, L., The ecology of rafting in the marine environment. II. The rafting organisms and community, Oceanography and Marine Biology: An Annual Review 43:279–418, 2005; epic.awi.de/11613/1/Thi2005a.pdf Return to text
- Gillespie, R.G. and Clague, D.A. (Eds), Encyclopedia of Islands, University of California, p. 302, 2009. Return to text
- Heads, M., Molecular Panbiogeography of the Tropics, University of California Press, Berkeley, CA, p. 416, 2012. Return to text
- Vitt, L.J. and Caldwell, J.P., Herpetology: An Introductory Biology of Amphibians and Reptiles, 3rd edn, Academic Press, San Diego, CA, fig. 18.3, p. 486, 2009. Return to text
- Vitt and Caldwell, ref. 48, fig. 18.22, p. 501. Return to text
- Vitt and Caldwell, ref. 48, fig. 20.26, p. 539. Return to text
- Elias, S. et al., Life and times of the Bering land bridge, Nature 382:60–63, 4 July 1996. Return to text
- Carranza, S. and Arnold, E.N., Investigating the origin of transoceanic distributions: mtDNA shows Mabuya lizards (Reptilia, Scincidae) crossed the Atlantic twice, Systematics and Biodiversity 1(2): 275–282 |doi: 10.1017/ S1477200003001099. Return to text
- Vitt and Caldwell, ref. 48, p. 520. Return to text
- Vitt and Caldwell, ref. 48, p. 489. Return to text
- Vitt and Caldwell, ref. 48, p. 564. Return to text
- Gillespie and Clague, ref. 46, p. 347–351. Return to text
- Ranidae, Hylidae and Bufonidae are disjunct across the Pacific but this could easily be explained by migration across a Bering land bridge. See note 59. Return to text
- Pyron, R.A., Biogeographic analysis reveals ancient continental vicariance and recent oceanic dispersal in amphibians, Systematic Biology 63(5):779–797, September 2014. Return to text
- Volcanic activity during the Flood would have warmed the oceans leading to a much warmer Bering land bridge arising from the surrounding warm water in the North Pacific and Arctic Oceans. Also the copious latent heat generated in the atmosphere from condensation would have contributed to mild winters. Return to text
- Oard, M.J., Frozen in Time: Woolly Mammoths, the Ice Age, and the Biblical Key to their Secrets, Master Books, Green Forest, AR., 2004. Return to text
- Statham, D.R., No evidence of evolution and ‘deep time’, Creation 35(4):40–41, October 2013. Return to text
- Feder, M.E. and Burggren, W.W. (Eds), Environmental Physiology of the Amphibians, University of Chicago Press, p. 527, 1992. Return to text
- Wells, K.D., The Ecology and Behaviour of Amphibians, University of Chicago Press, Chicago, IL, pp. 114–116, 2007. Return to text
- Schmidt–Nielsen, K., Animal Physiology: Adaptation and Environment, 5th edn, Cambridge University Press, Cambridge, p. 323, 1997. Return to text
- Savage, J.M., The geographic distribution of frogs: patterns and predictions, in Evolutionary Biology of the Anurans, Vial, J.L. (Ed.), University of Missouri Press, Columbia, MO., p. 366, 1973. Return to text
- tolweb.org/Pipidae/16986; pddb.org. Return to text
- Olori, J.C., Tree of Life Web Project, tolweb.org. Return to text
- Savage, ref. 65, p. 357. Return to text
- The newt and salamander portal; caudata.org. Return to text
- Savage, ref. 65, p. 358. Return to text
- Lomolino, M.V. et al., Biogeography, 3rd edn, Sinauer Associates, Sunderland, MA., p. 160, 2006. Return to text
- George, W. and Lavocat, R. (Eds), The Africa–South America Connection, Clarendon Press, Oxford, ch. 8, 1993. Return to text
- George and Lavocat, ref. 72, ch. 9. Return to text
- tolweb.org/Noctilionoidea/16093. Return to text
- Allaby, M., Dromiciopsia, A Dictionary of Zoology, Oxford University Press, Oxford, 1999. Return to text
- Crottini et al., Vertebrate time–tree elucidates the biogeographic pattern of a major biotic change around the K–T boundary in Madagascar, PNAS 109(14):5358–5363, 3 April 2012 | doi: 10.1073/pnas.1112487109; pnas.org/ content/109/14/5358.full. Return to text
- Noonan, B.P. and Chippindale, P.T., Vicariant origin of Malagasy reptiles supports late Cretaceous Antarctic land bridge, The American Naturalist 168(6):730–741, December 2006. Return to text
- Ali, J.R. and Aitchison, J.C., Kerguelen Plateau and the Late Cretaceous southern–continent bioconnection hypothesis: tales from a topographical ocean, J. Biogeography 36:1778–1784, 2009. Return to text
- Ali, J.R. and Krause, D.W., Late Cretaceous bioconnections between Indo– Madagascar and Antarctica: refutation of the Gunnerus Ridge causeway hypothesis, J. Biogeography 38:1855–1872, 2011. Return to text
- Briggs, J.C., Biogeography and Plate Tectonics, Elsevier Science, New York, pp. 118–119, 1987. Return to text
- Darlington, ref. 20, p. 545. Return to text
- De Queiroz, ref. 7, p. 248. Return to text
- Ali, J.R. and Huber, M., Mammalian biodiversity on Madagascar controlled by ocean currents, Nature 463:653–656, 4 February 2010. Return to text
- Kier, G. et al., A global assessment of endemism and species richness across island and mainland regions, PNAS 106(23):9322–9327, 9 June 2009. Return to text
- Lomolino et al., ref. 71, pp. 651–652. Return to text
- Humphries and Parenti, ref. 3, p. 87. Return to text
- Catchpoole, D. and Wieland, C., Speedy species surprise, Creation 23(2): 13–15, March 2001. Return to text
- Wise, K. and Croxton, M., Rafting: a post–Flood biogeographic dispersal mechanism, Proceedings of the Fifth International Conference on Creationism, Creation Science Fellowship, Pittsburgh, PA, pp. 465–477, 2003. Return to text
- Batten, D. (Ed.), The Creation Answers Book, Creation Book Publishers, ch. 17, 2009. Return to text
- Woodmorappe, J., Causes for the biogeographic distribution of land vertebrates after the Flood, Proceedings of the 2nd International Conference on Creationism, Pittsburgh, PA, pp. 361–267, 1990. Return to text
- Humphries and Parenti, ref. 3, p. 21. Return to text
- Myers, N. et al., Biodiversity hotspots, Nature 403:853–858, 2000. Return to text









Readers’ comments
Comments are automatically closed 14 days after publication.