Life in a test-tube
For decades, Hollywood has been presenting images of ‘Martians’ and ‘space creatures’ through movies such as Independence Day and Alien. Behind this is the idea of evolution: if life evolved on earth, then why couldn’t it have evolved on other planets? One famous experiment is widely touted as proof that life could have evolved from non-living chemicals.
Miller’s experiment
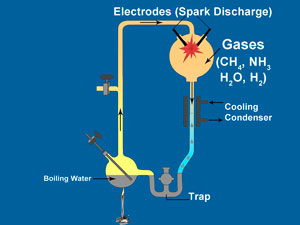
In 1953, the same year that DNA’s double helix structure was discovered, a young graduate student named Stanley Miller sparked some gases and formed amino acids. These are the building blocks of proteins, a major component of living cells. So thousands of newspapers worldwide erroneously reported that he had, in essence, created life in a test-tube. This experiment became textbook orthodoxy.
However, textbooks tend to present alleged ‘proofs’ of evolution without critical discussion. Unless students consult outside sources, they often over-value the connection between organic molecules and life. Bold claims such as ‘organic molecules could have arisen on a lifeless Earth’ tend to mislead students into believing that organic molecules are life. However, ‘organic’ does not mean the molecules are alive, but simply refers to any molecule that contains the element carbon.
Some of the organic compounds of significance to the origin of life are amino acids and sugars. For life to exist, these and other non-living components must be arranged in a special way. The difference between living and non-living things is not so much the substances they contain, but how these substances are organized.
But can non-living chemicals organize themselves into the sorts of precise sequences that we observe in living systems today? Did Miller’s experiment really show that matter has this capability? Thinking in purely naturalistic terms, let’s try to answer that question. How could the first cell have originated in the hypothetical ‘prebiotic soup’? What would have been the first sub-units formed that later might have given rise to the first cell?
The Miller experiments created a few of the more simple amino acids and other simple compounds by discharging electrodes into a mixture of methane, ammonia, water vapour and hydrogen. Does this mean that perhaps amino acids could have been the first components of the cell to form? Absolutely not. The first thing to keep in mind is that laboratory experiments often differ from the real world in a number of ways.
Chemical problems
To begin with, Miller’s experiment used a flask equipped with a trap to collect the amino acids. In textbook accounts, the trap is only described in passing, as it was part of the apparatus he used. It is almost never mentioned that the trap served to protect the amino acids from the same energy that was used to create them.
In fact, this energy would be many thousands of times more effective at destroying these molecules than forming them. Some have proposed that tide pools, lakes or clays may have served as traps on the early earth. ‘But solving the trap problem would make another problem, because the molecules that must be protected from energy sources also need that energy to advance to the next stage. Thus the idea of a trap actually would be fatal to evolutionary theory.’1
But what if both amino acids and sugars were somehow able to beat all the incredible odds and were not only able to exist, but to exist together? Dr Duane Gish, who has extensive experience in forming proteins, says,2 ‘When amino acids and sugars are together they combine so readily that they cancel each other out. … When an amino acid and sugar bind in this way, the product is neither a sugar nor an amino acid. That is, they chemically combine and destroy one another.’3
Gish says it is generally assumed that more amino acids than sugars existed on the early earth. If this is the case, this would not help evolutionists. All the amino acids would react with the available sugars and there would be no sugars left to create the ‘backbone’ of the vital coding molecule, DNA.
But let’s give the evolutionists the benefit of the doubt. Let’s concede that sugars were somehow available and DNA formed. What would have happened to it? ‘Most evolutionists believe life first appeared in the ocean,’ says Gish. ‘However, in water, nucleotides [individual units of DNA] don’t come together but break apart through a process called hydrolysis. In this process, energy is released. This is the opposite of what’s needed. To bring a DNA molecule together, tremendous energy must be poured in to force those chemical bonds to form.’
But for nucleotides to bond in the right way, they need energy as well as at least 100 enzymes (a type of protein) working together. This presents a ‘catch-22’ situation. The enzymes are needed to form DNA by combining its nucleotides, yet these enzymes cannot be formed unless the DNA codes for them.
To suggest that one or two sub-units could have existed before the presence of a living cell is totally fallacious. Rather, the living cell functions by an interdependence, where one part depends on another.
Problems ignored
Any search for a naturalistic explanation to the origin of life must confront and try to answer the above unanswerable obstacles. Not surprisingly, these incredible complications are censored from almost all biology textbooks. If this critical information was included in biology curricula, the vast majority of students would have to conclude that matter could not have changed itself into living things.
Furthermore, they would come to this conclusion based on what they know and can see about the properties of matter. God never intended our plain and obvious origin with Him to be a mystery. God said that we would clearly be able to see His power and divinity in nature (Romans 1:20). The origin of life is mysterious only to the one who has been exposed to all the facts, but ignores them in the hope of finding a ‘God-free’ solution.
Telling your left hand from your right

Another often unmentioned fact of Miller’s experiments is that two types of amino acids were created—they are mirror images of each other, just like your two hands. Living systems are composed of only ‘left-handed’ amino acids. However, in Miller’s experiment, and in any natural process, a 50:50 mixture of left-and right-handed amino acids is produced. When both types of amino acids are present, they chemically combine with one another in ways that make the resultant protein totally inactive. Left to themselves, the amino acids produced in Miller’s flask were nothing but evolutionary dead-ends.
Miller’s experiment failed to demonstrate how life’s exclusive preference for left-handedness could have been achieved naturalistically. In an extensive interview, Dr Gish explained how the natural behaviour of organic molecules poses insurmountable problems for a naturalistic origin of life. ‘Without the presence of DNA to direct the order of amino acids,’ says Gish, ‘the natural tendency is that both types of amino acids would combine equally well with one another. There is no tendency for left-handed ones to react only with left-handed ones.’1 By this he meant that the normal tendency would be that perhaps two or three left-handed amino acids might attach together, then a right one, then maybe a left one and so on. He says the problem with the presence of both types is that normal proteins contain, on average, 400 amino acids. This means that by chance, 400 or so left-handed amino acids would have had to come together without any right-handed ones. ‘If we had a protein with 400 amino acids,’ Gish explains, ‘the probability of only all left-handed amino acids is ½ times itself 400 times (½400 ) [or one in 4 x 10121 , which is a number so large that it would be written as 4 followed by 121 zeroes]. If just one right-handed amino acid were to bond with any of the other 400 left-handed amino acids, it would render the whole chain inactive. It becomes inactive because right-handed amino acids alter the shape of the chain. An enzyme [a special type of protein] must fold in a very precise way in order to allow a substrate to attach to it. If a substrate cannot attach to the enzyme, it becomes useless.’
Perhaps such useless proteins would have had a better chance if DNA had formed first. Perhaps the sugars that have been created in other origin-of-life experiments could have come together with phosphates and bases to produce the DNA. Like amino acids, sugars occur in left-and right-handed forms. However, only right-handed forms of sugars are found in living systems. The problem with the presence of both forms is that it would prevent two complementary strands of DNA from coming together. On this particular point, Gish makes a useful analogy, ‘Imagine a string of right-handed gloves tied end-to-end and you want to insert a series of right hands tied from end-to-end, but one of the hands was a left hand. The two could never come together.’ If two complementary strands of DNA could not be joined, then it would never conform into a double helix shape. Without this shape, DNA could not replicate or perform any of the functions it now has.
Note
- Personal interview with biochemist Dr Duane Gish, 13 May 1996.
Re-posted on homepage: 19 April 2017
References and notes
- Bliss, R., Parker, G. and Gish, D., Origin of Life, CLP Publishers, California, USA, 1990. Return to text.
- Personal interview, 13 May 1996. Dr Gish has a Ph.D. in Biochemistry, University of California, Berkeley, and was Vice President of the Institute for Creation Research in San Diego. Return to text.
- The amino group (–NH2 ) in the amino acid reacts readily with the carbonyl group (O=C<) in the sugar, releasing a water molecule (H20) to form an imine (HN=C<), which is useless for life. Return to text.








Readers’ comments
Comments are automatically closed 14 days after publication.