Species were designed to change, part 3
The tangled web of (intrabaraminic) life
In Species were designed to change, part 1, I outlined the many different ways that God engineered life to adapt and change. In part 2, I covered the formation of new species and how this fits beautifully into the creationist concept that God front-loaded the created kinds with a significant amount of potential for change. I would now like to turn your attention to a concept that will seem rather strange for many readers.
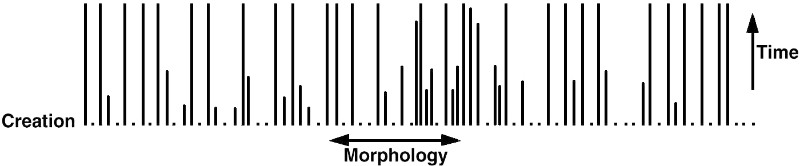

Most people familiar with modern creationist writing will have heard us make a distinction between the ‘creationist lawn’ (figure 1), which is clearly false, and the ‘creationist forest’ (figure 2), which is a better description of what we expect to see. Anti-creationists try and stick us with the thought that God created all species the same as they are today. This is nonsense, as the first two parts of this series clearly showed. There is no reason to believe that all species can be represented by straight lines over time (like blades of grass in a lawn). Instead, we have long taught that it is more accurate to depict ‘created kinds’ that break up into different species over time. This can be depicted like branching trees in a forest, with each trunk representing a created kind and the various branches representing the different species that have come from that kind.
A net-like pattern of interconnected species
Yet, the creationist forest concept is still incomplete. It does not fully encapsulate what we see, and it might give people the wrong impression that ‘species’ are discrete. I would like to suggest we think of the species within each baramin more like a web, a net, or a meshwork (figure 3). Thus, I am using this article to introduce the ‘braided baramin’ concept.

I was first introduced to this idea in graduate school when we discussed a book titled Corals in Space and Time1 by the well-known coral reef scientist J.E.N. Veron. He used the phrase ‘reticulate evolution’, where ‘reticulate’ means net-like. The concept is simple enough even if the writing in the book was a bit cryptic (there were several points where nobody in the class, including the professor, could figure out the grammar).
Veron claimed that evolution in corals happens with an interconnected pattern of speciation over time and space. If you go to a coral reef today, for example, you might find many species in the genus Acropora. But if you go to another reef, you may not find the same species. In fact, you might come across a species that looks intermediate between those on another reef. If you travel back in time, the fossil record displays many more intermediate-looking species. It is easy for a trained person to quickly identify the family to which a living or fossil species belongs. The exact species, however, can often be quite hard to figure out. Even the experts argue over some species definitions. My first published scientific paper dealt with this issue,2 and the three coral species I studied have since been moved into a new genus.
I spent a lot of time in graduate school studying coral reproduction in the Florida Keys. Almost all the species of coral in genus Acropora are broadcast spawners. On one or two nights a year, a few hours after sunset, each coral colony from many different species will release thousands of egg-sperm bundles into the water column in a mass-spawning event (figure 4). This is one of most amazing wonders of the natural world and anyone who has witnessed it is truly privileged.
These bundles float to the surface and break apart, at which point the sperm from one colony seek out and fertilize the eggs from another. There are only three species in this genus in the Caribbean, and one (Acropora prolifera) is a hybrid of the other two (A. palmata and A. cervicornis) that regularly back-crosses with its two parent species.3 In the Pacific Ocean, however, there are hundreds of species in this genus living (sometimes literally) on top of each other. If corals from many different species are spawning at the same time, how is it that they don’t all just fertilize each other? How do they maintain species boundaries? It turns out that there are special recognition factors on the egg’s surface. Only those sperm with the correct ‘key’ can fertilize the egg, and the lock-and-key mechanism varies from one species to another. It can even vary within a species, meaning that some colonies will be able to reproduce with colonies from other species. Variations among the genes that code for these recognition factors help to maintain species boundaries, but these variations also create an almost bewildering pattern of cross-species hybridization. The relationships among the many species forms a braided, net-like pattern.

Putting aside the evolutionary spin, reticulation is a perfect analogy for the concept I am trying to introduce here. After God created each individual baramin, as time passed, smaller groups split off to form what we call ‘species’. These were not necessarily reproductively isolated from other members of the baramin, however, so we might see different species ebbing and flowing through the fossil record. We might even see species merging and separating today, like has been observed among Darwin’s finches on the Galápagos islands.
Hybridization among species should not be a surprise to us. Species remaining distinct even though they can potentially mix with other species should also not be a surprise. There are all sorts of reasons why species distinctions can be maintained, including mate choice, differences in reproductive timing, geographic separation, size or some other geometric incompatibility, differences in egg-sperm recognition factors, or a lack of shared synteny (the order of genes along chromosomes). Yet, when we look at each of the created kinds, we see that some have many species today and some have but a few. The extremes can be seen when you compare mice (genus Mus), of which there are dozens of species and innumerable subspecies, and the duckbilled platypus (Ornithorhynchus anatinus), which is the only living species in its genus, and the only living genus in family Ornithorhynchidae.
Classifying baramins
God created distinct ‘kinds’ in Genesis. When discussing the kinds, creationists often use the word baramin, which I have already done, combining the Hebrew words for create (bara) and kind (min).
Given a created kind (baramin), God could have created few to many individuals. He could have front-loaded those individuals with few to much genetic diversity and with a small to large capacity to change over time. A created kind could have a single main trunk or many. Confused? Perhaps breaking this down into smaller bits will help. We can divide baramins into several classes or types (Table 1). There is a lot of overlap in the following definitions but consider these as discrete categories for the time being.
Table 1: Several baramin classes.
| Type | Subtype | Diversity | Starting Number | Description |
|---|---|---|---|---|
| 1 | -- | Limited | Two | Humans |
| 2 | a | Low | Few | Low diversity baramins, initially in specific pockets |
| b | Low | Many | Low diversity baramins, widely dispersed | |
| 3 | a | High | Few | High diversity baramins, initially in specific pockets |
| b | High | Many | High diversity baramins, widely dispersed | |
| 4 | -- | Low | Many | Purely asexual species |
The Type 1 baramin

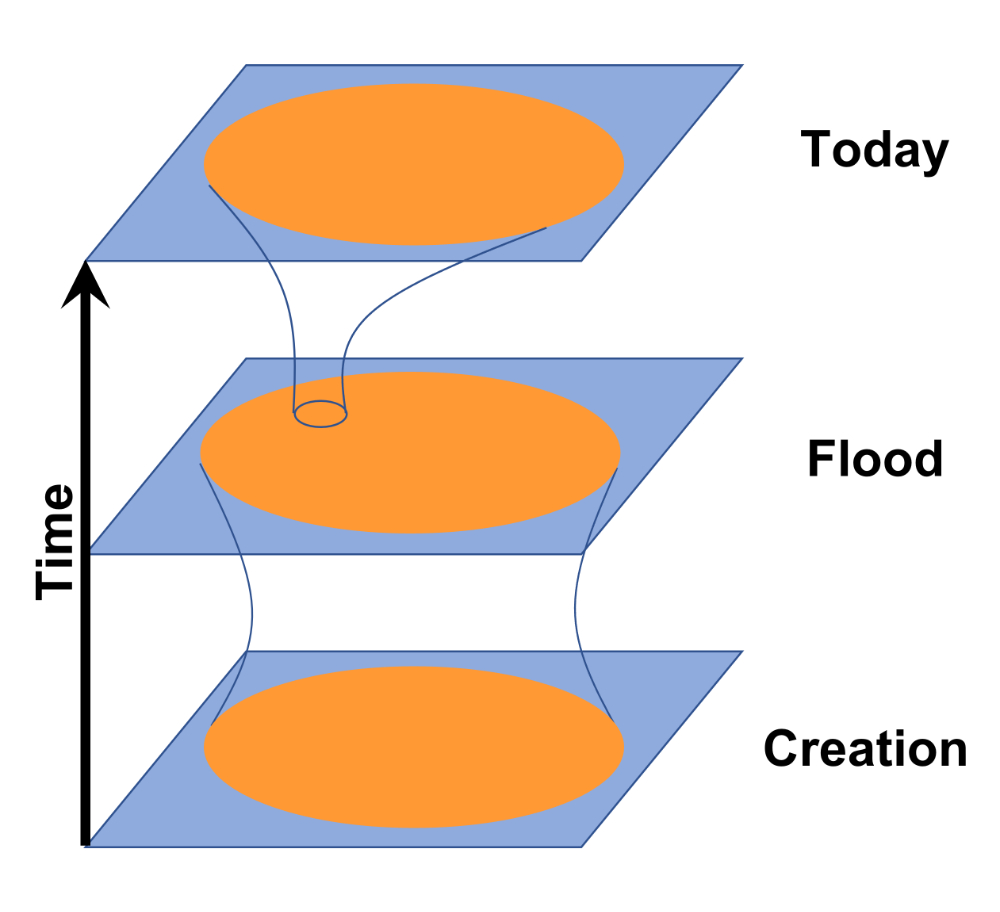
Humans are the only known baramin to have been created with just two founding individuals. All people who have ever lived, including ancient people like Neanderthals and Denisovans, descend from Adam and Eve. This puts us in a unique class which I am calling the Type 1 baramin (figure 5). Let us not, however, think that humans should have only a little genetic diversity. God could easily have front-loaded Adam’s genome with much more heterozygosity than the average person carries today. Eve could also have had a completely different genome. Adam and Eve could also have been engineered with multiple different genomes in their reproductive cells.4 In the end, the amount of genetic diversity we see in humans, both today and in the fossil record, would be a product of how many children Adam and Eve had. Every other person in the human family (with the exception of Jesus) came about through the normal process of sexual reproduction, but we have the capacity to carry quite a bit of diversity even if our family tree started from a single, narrow trunk.
The Type 2 baramin
Consider a group of animals that are all genetically similar, like a flock of white sheep. Generations of selective inbreeding have led to the loss of most of the genetic diversity in the flock. Black sheep can never appear among them because the genes for other coat colors are simply not there. If you were to start a new flock by taking just a few of these animals, you would not have much genetic diversity. Yet, if you started a new flock by pulling out a larger group of animals, you would not have much more genetic diversity. In fact, it would be difficult to tell how many animals you started with because the diversity in the original flock is so low.
In a similar way, God could have created some baramins with low initial diversity. This is a Type 2 baramin.
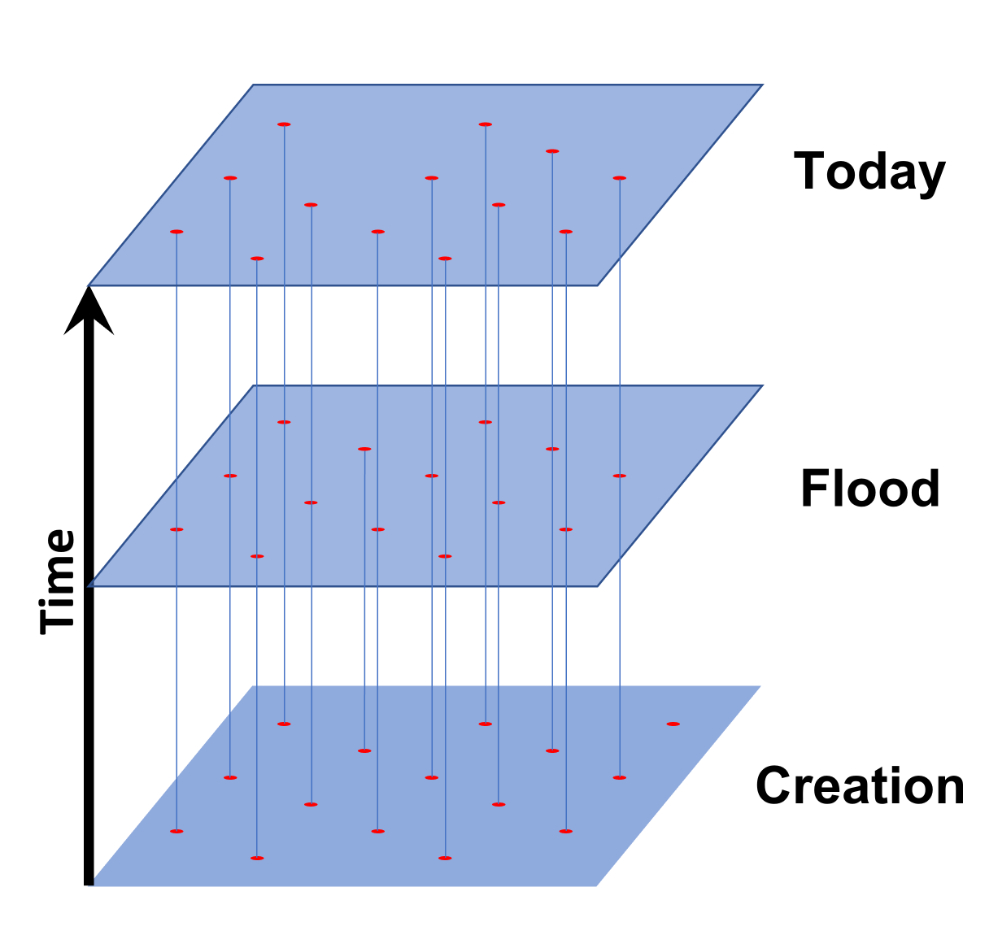
Yet, since He was not limited to creating only two of each kind, we have several options. A Type 2a baramin is a low diversity, sexually reproducing group which started with only a few individuals. A Type 2b baramin (figure 6) is one which started with more than two individuals, perhaps many. It might be possible to use sophisticated statistics to separate the two subtypes, but since creation was many thousands of years ago, this will be difficult.
I am describing the different baramin categories as they stood at the end of Creation Week. Hence, the diversity of fossils that were buried in the Flood might tell us something about the starting state of each representative baramin. After the Flood, things will be different. All unclean animal baramins were reduced to Type 1 baramins (two starting individuals) by default. There were more clean animals within each kind on the Ark, but since they tend to be kept in isolated flocks, this does not guarantee there was more genetic diversity among them.
Created diversity, in both active and latent forms (discussed in part 1 of this article series), could easily lead to ‘speciation’ in Type 2 baramins over time. However, Type 2 baramins are more limited than what follows.
The Type 3 baramin
Consider the possibility that God created a world brimming with life. The animals that Adam named in the Garden were not necessarily the only ones alive. It was not like Adam saw the only two members of the dog kind and decided to call one “Fido” and the other “Fifi”. Instead, he named them ‘dogs’ (in whatever language he was speaking). Later, if he ran into more members of the dog kind outside the Garden, he would still have called them ‘dogs’. Yet, the new animals did not necessarily have to look like the first ones he saw. They could have been a different size, different colors, etc. They would have all had a certain ‘dogness’ about them, but a Type 3 baramin has a tremendous potential for variation because it starts with many initial members who have high amounts of genetic diversity among them. A Type 3 baramin has multiple starting trunks. Yet, since the representatives all belong to the same baramin, the trunks can merge and split over time. This gives us a recipe for maximal speciation. It also give us the ‘braided baramin’ concept.
It might be easier to get your mind wrapped around this if you stop thinking about vertebrate animals. Most people have this vague idea that there were but two of each of the created kinds, but the requirements of a worldwide ecology tell us that there must have been more than two microscopic nematodes living in the soil, two green alga stalks living in the seas, and two tufts of grass poking out of the ground. What about beetles, butterflies, worms, and wasps? Did God only create two of each and put them in the Garden? Instead, it is likely that He peppered the created world with untold riches of genetic diversity within multiple kinds. Also consider that most of the created kinds did not have to be taken on the Ark. Most of the kinds Noah rescued were what we would call terrestrial vertebrates. Plants, fish, and the many invertebrates do not count.

We can divide Type 3 baramins into several subtypes depending on how much connectivity was found within them at Creation. Consider a snail baramin that lives along rocky coastlines only. If the places it likes to inhabit are few and far between, there would naturally be reproductive isolation among the various pockets in which this baramin is found. Yet, if members of different populations do eventually meet and hybridize, a burst of speciation might occur as the genes for the two different populations are mixed. I call these Type 3a baramins (figure 7). Biologists use the term syngameon to denote a group of interrelated species that have few reproductive barriers and high rates of hybridization. This is very similar to the concept of a Type 3a baramin. However, consider plants that reproduce via wind-blown pollen or birds that migrate great distances. It would be difficult to maintain reproductive barriers within those baramins. A Type 3b baramin is a widespread, genetically diverse population that could be represented by a single species or a species syngameon, depending on where a person wants to draw the line between Type 3a and 3b baramins.
The Type 4 baramin
All of the baramins categorized above explain sexually reproducing organisms. There are many other organisms that alternate between asexual reproduction and sexual reproduction, including bacteria, which often exchange DNA with one another. There are many other organisms that seem to have lost the ability to reproduce sexually, including examples among the reptiles, fish, and crustaceans. Purely asexual reproduction is incredibly rare among multicellular animals (bdelloid rotifers are one of the exceptions). Yet, we must allow for the existence of some purely asexual baramins that were designed that way in the beginning and that have either maintained that status since they were created or gone extinct. This would give us a Type 4 baramin (figure 8). Since there is no mixing of genes from one generation to the next, speciation rates in purely asexual baramins would be quite low even if their numbers can be quite high.
What is a species, anyway?
Classifying baramins in this way helps us to resolve a tremendous difficulty in biology: the lack of a definition of the word ‘species’. The Latin word simply means “type.” This is not very helpful because what separates one ‘type’ of organism from another is very much in the eye of the beholder.
I spent several years in college helping to identify insects living in streams for my state’s environmental protection division. I could easily identify the caddisflies, mayflies, stoneflies, etc., and I could usually assign most of my specimens to the species level. I distinctly remember one of my more experienced coworkers gladly rejoicing when he found an insect larva with three hairs on its forecoxa instead of the usual two! He triumphantly announced that he had found a new species in that stream. Yet, without a detailed knowledge of the genes that code for the number of hairs on the legs of a baby mayfly, the frequency of those genes, or the mating habits of mayflies with one extra hair on their front leg, how could anyone know? This illustrates a great difference between the ‘lumpers’ and the ‘splitters’ among taxonomists. I am a lumper. The concept of braided baramins adds to this.
Defining species
Paleontologists use the word ‘species’ to simply indicate things that look different. Because they never deal with anything that is actually alive, they have no idea if those organisms could mate and produce offspring. They also generally ignore the great variety we see among creatures that can mate. A species, to a paleontologist is something they can readily identify in the rocks. This has nothing to do with biology, yet paleontology has a profound influence on the beliefs of biologists.
Sadly, biologists also do not have a good definition of the word. It can refer to a group of organisms that breeds true to type, a group of organisms that can successfully interbreed regardless of what they look like, etc. Ecologists are often tempted to define the word even more narrowly. After all, if an impassable mountain chain separates two herds of caribou, we may as well treat them as different species, right?
How does the Type 3 baramin concept help us to define species? If God had created a bewildering array of genetic factors within a Type 3 baramin, it could easily contain a wide range of phenotypes. Those phenotypes could be scattered in pockets across the landscape (or ocean-scape) in the antediluvian world, only to be buried chaotically in the worldwide Flood. How could a paleontologist make heads or tails of such a situation? During the Flood, the baramins not carried in the Ark would have suffered from massive die offs, but those that did survive would go on to repopulate the land and sea, probably starting in discrete little pockets and spreading outward from there. When members of the same baramin that arose from different founder events met, they could either hybridize, which could potentially unleash another wave of ‘speciation’, or live side-by-side as discrete species.

Consider trilobites (Kingdom Animalia, Phylum Arthropoda, Class Trilobita). On the order of 22,000 trilobite species have been described in the scientific literature, but a consistent classification scheme has eluded researchers. Over the years, trilobite taxonomy has undergone several major revisions. The history and confusion of trilobite classification was recently reviewed by Paterson (2019).5 The latest comprehensive scheme (Adrain 20116) breaks them up into eleven distinct orders but sets aside 58 trilobite families that cannot be assigned to any specific order. If we had DNA, perhaps trilobite classification would be easier, but DNA has often overturned carefully organized evolutionary schemes that were based on morphology alone. The few examples of preserved trilobite soft tissue that have been found were associated with surprising connotations (i.e. the results were not what they were expecting). Are trilobites a perfect example of a massive and complex Type 3b baramin? Or did God create multiple trilobite baramins? I suspect the latter. The complex interrelationships among the species, genera, and families leads me to think there are multiple Type 3b baramins here. Taxonomy is not easy. Our God is a God of diversity and complexity.
In the end, species are transitory things. A butterfly with blue wings can become a butterfly with black wings. Polar bears can merge with brown bears. Turtles with medium necks can change into turtles with short necks and turtles with long necks. This dance of disappearing species has continued from Creation to the present. Darwin was on a fool’s errand, trying to pinpoint the origin of something ephemeral. Instead of searching for the origin of species, whatever that word even means, he should have been looking for the origin of created kinds. Yet, he knew the fossil record of his day did not have evidence of the major evolutionary transitions. Instead, it reflected discrete categories of living things, as if one kind did not evolve into others. He was looking at the data from the wrong end.
A proper understanding of the baramin concept allows us to explain the appearance and disappearance of species over time, the lack of major transitions in the fossil record, and the reason why so many ‘species’ can interbreed today.
‘Change over time’ is not the same thing as ‘evolution’
Our opponents think they have backed us into a corner because as soon as we say we believe in ‘change over time’. They reply, “Aha! Then you believe in evolution!” Many people are intimidated at this point, but the argument only works if you adopt the lamest possible definition of evolution.
Yes, the word ‘evolution’ means ‘change over time’. The concept, however, involves much more than that. For example, what if the change goes in the wrong direction (i.e. ‘downhill’) or what if the changes are cyclical, going back and forth? This is NOT what they mean. Instead, following Darwin, they take the idea that species change and add to it the thought that any change is possible, given many millions of years. Darwin wrote, “I can see no limit to the amount of change.”7 Thus, his imagination allowed him to believe in evolution, not the data. You see, evolution is a belief that enough change over enough time can lead to the common ancestry of all species.

‘Change over time’ is a lame definition of evolution because it leaves out the part about common ancestry. Change over time is necessary, but we believe the changes we see are not sufficient to explain common ancestry (figure B1). This is the essence of the creation evolution debate. We should not be afraid of ‘change over time’ because it is something God directly engineered into creation.

Also, if both sides claim ‘change over time’ is part of their model, clearly this is not proof of evolution and disproof of creation. Instead, it becomes what I call “non-discriminating information”, or information that cannot be used to make a case for either of two competing theories, as I explained in How to Think, Not What to Think (figure B2).
References and notes
- Veron, J.E.N. Coral in Space and Time: the biogeography & evolution of the Scleractinia, Cornell University Press (Ithaca, NY), 1995. ISBN: 0-801-48263-1. Return to text.
- Manica A. and Carter, R.W., Morphological and fluorescence analysis of the Montastraea annularis species complex in Florida, Marine Biology 137:899–906, 2000 | doi:10.1007/s002270000422. Return to text.
- Precht, W.F., Vollmer, S.V., Modys, A.B., and Kaufman, L. Fossil Acropora prolifera (lamarck, 1816) reveals coral hybridization is not only a recent phenomenon, Proc Biological Society of Washington 132(1):40–55, 2019; | doi: 10.2988/18-D-18-00011. Return to text.
- Sanford, J., Carter, R., Brewer, W., Baumgardner, J., Potter, B., and Potter, J., Adam, Eve, designed diversity, and allele frequencies; in: Whitmore, J.H. (Ed.), Proceedings of the Eighth International Conference on Creationism, Creation Science Fellowship, Pittsburgh, PA, pp. 200–216, 2018. Return to text.
- Paterson, J.R., The trouble with trilobites: classification, phylogeny and the cryptogenesis problem. Geological Magazine 157 (special issue 1):35–46, 2019. Return to text.
- Adrain JM (2011) Class Trilobita Walch, 1771. In Animal Biodiversity: An Outline of Higher-Level Classification and Survey of Taxonomic Richness (ed. Z-Q Zhang). Zootaxa 3148, 104–9. Return to text.
- Darwin, C.R., On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life, 1st ed., John Murray, London, p. 109, 1859; darwin-online.org.uk Return to text.














Readers’ comments
Comments are automatically closed 14 days after publication.