Journal of Creation 34(2):90–97, August 2020
Browse our latest digital issue Subscribe
Rapid growth of caves and speleothems:
part 2—growth rate variables
Although growth rates depend on many variables, there are five main ones that determine the rate of carbonate growth of speleothems. First, the most significant variable is the concentration of calcium ions in the drip water and the resulting supersaturation that occurs when the drip water enters the cave. This depends upon the amount of soil carbon dioxide that is dissolved into the soil water. As the soil water percolates through the carbonate, carbonic acid slowly dissolves the rock. Second, cave temperature has a major effect, and depends largely upon the surface air temperature. Third, cave air ventilation is also an important factor. Better ventilation increases the growth rate of a speleothem. Fourth, rapid speleothem growth generally requires a higher drip rate. Fifth, the thickness of the water film on top of the stalagmite (which is increased by a higher drip rate) is another major variable. In addition to these five generally recognized variables, evaporation is a significant variable at Carlsbad Caverns, which is well ventilated. It could have been a significant variable for other caves, as well.

The variables needed for speleothem growth
Many variables and complex processes determine the growth or dissolution of speleothems.1,2 Research has been conducted on stalagmites because stalactites, being located on the cave roof, are difficult to study (for obvious reasons). The main variables for the growth of a stalagmite are: (1) concentration of the drip water Ca2+ ion; (2) cave temperature; (3) cave atmospheric partial pressure of CO2; (4) drip water flow rate; and (5) the thickness of the thin film of water flowing over stalagmites (table 1).3,4 In addition to these five variables, another will be briefly discussed and that is evaporation, which for most caves is now insignificant, but could have been significant during the Ice Age. All of these variables depend upon many other variables, which makes the use of stalagmites as records of paleoclimate difficult. However, this is a major reason why so much research and literature exist on cave speleothems.5-9
Table 1. The five main variables that determine stalagmite growth.

Researchers also measure many trace elements, such as Mg and Sr, and isotope ratios, such as δ18O and δ13C, within the stalagmites. They believe these trace elements give them more information about the conditions for carbonate deposition.10 Nonetheless, there are many other variables involved11 that can cause misinterpretation. Impurities within the drip water can inhibit calcite deposition,46 such as Mg, which can be common in carbonate that contains a fair proportion of dolostone. Moreover, aragonite commonly forms instead of calcite in carbonates that have a higher proportion of dolostone,12 and aragonite, since it is metastable, can transform into calcite with time.
Isotope ratios are thought to be related to the paleoclimate, such as glacial/interglacial oscillations in the uniformitarian ice age paradigm. However, both δ13C and δ18O in speleothems can result from many variables, which makes paleoclimate interpretations difficult.13 Cheng et al. write:
“Although the speleothem δ18O signatures can be influenced by numerous and complex factors … . However, the climate interpretation of δ18O records remains a subject of considerable debate, particularly in the EASM [East Asian summer monsoon] domain.”14
I will discuss the five main variables, and some of their related variables. We need to understand how speleothems grow in order to show in part 315 that the very slow calcite deposition rates today would have been greatly accelerated during the post-Flood Ice Age.
The drip Ca2+ ion
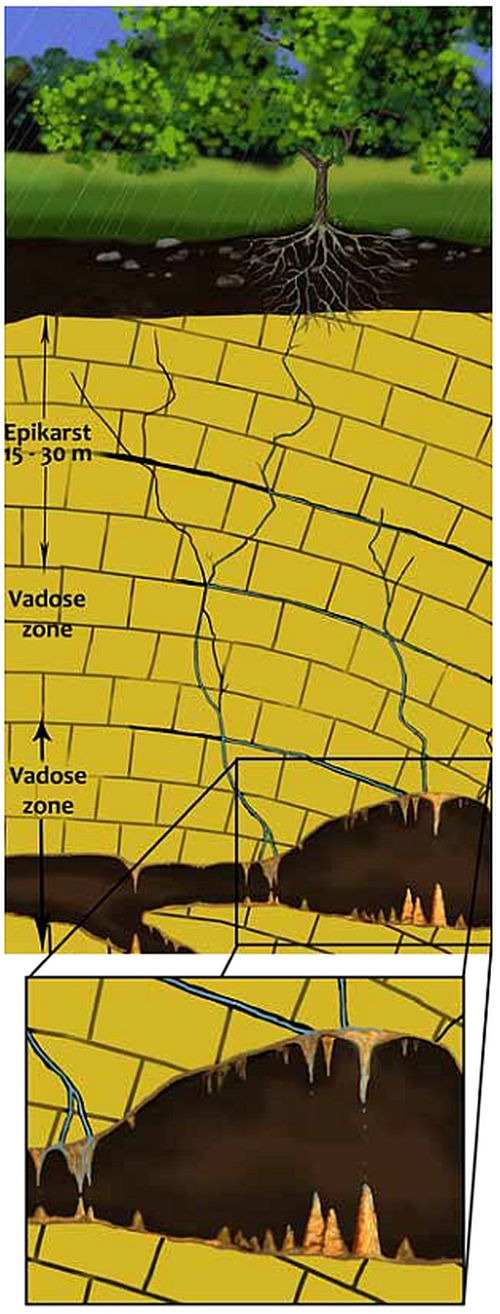
Of the variables above, the concentration of the Ca2+ ion in the drip water is the most important for calcite deposition on speleothems.16 This depends primarily upon the partial pressure of the soil CO2 and any changes in the water that percolates through the top of the carbonate before entering the cave (figure 1). The partial pressure of soil CO2 in turn depends upon more variables.17 The carbonate layer just below the soil is generally called the epikarst and is typically 15 to 30 m (50 to 100 ft) thick. The rest of the carbonate above the cave is called the vadose zone in which air fills the cracks and voids. It is the zone above the water table, which includes the epikarst. The phreatic zone is the zone below the water table, which is normally below cave level. The following are the basic equations in the conversion of atmospheric CO2 to carbonic acid in the soil or epikarst:18
CO2(g) ↔ CO2(aq) (1)
H2O + CO2(aq) ↔ H2CO3(aq) (2)
H2CO3(aq) ↔ H+ + HCO3(aq)- (3)
HCO3(aq)- ↔ H+ + CO3(aq)2- (4)
where the “(g)” means the gaseous state and “(aq)” means dissolved in the water. Equation (1) is the rate limiting process that slows down the other conversions in equations (2) to (4), which proceed rapidly to equilibrium.19 Equation (3) represents the dominant end process with equation (4) a minor variant. Only a small proportion of the CO2 is converted to carbonic acid.20 Much CO2(g) escapes the soil into the air, which interestingly is 10 times the emission of CO2 to the air as from fossil fuels.21 Moreover, all these reactions are reversible and go in the direction from the high-concentration species to the low-concentration species until equilibrium is reached. In other words, if the groundwater is high in CO2(aq), the reaction will go towards H2CO3(aq) and finally to H+ + HCO3(aq)-. So, the groundwater becomes mildly acidic forming carbonic acid when CO2 is added to the groundwater.
When this water begins to seep down through the cracks in the epikarst, it dissolves the calcite (CaCO3) and gives off CO2 to the water or air in the vadose zone. The downward-seeping water quickly becomes saturated with Ca(aq)2+.
When the water enters the cave chamber with lower partial pressure of CO2 than the drip water, CO2 escapes from the water according to the following reaction:
Ca(aq)2+ + 2HCO3(aq)- ↔ CaCO3 + CO2(g) + H2O (5)
The drip water is generally neutral upon entering the cave, but quickly becomes supersaturated upon the degassing of CO2 (figure 2). Nonetheless, this reaction is also reversible, as equation 5 shows. When a lot of calcium and carbonic acid is in the drip water, the reaction goes to the right and speleothems grow, proportional to the concentration of Ca2+. But, if there is little Ca2+ in the water (undersaturated), the reaction goes to the left, dissolving calcite on the speleothems. In carbonate caves, the reaction almost always goes to the right. As such, it is the degree of Ca2+ supersaturation upon CO2 degassing that determines the growth of speleothems.

However, the excavation of the cave opening would require undersaturation, if it were really dissolved by carbonic acid (which it was not22), while deposition requires supersaturation. As more CO2 is liberated into the cave air, the drip water becomes progressively more supersaturated. Thus, the greater the supersaturation, the faster the speleothem growth. Still, deposition of calcite does not automatically happen when supersaturation first occurs. It must first reach a threshold of about 10% before calcium carbonate can be deposited on speleothems.23 However, if other elements such as Mg are present, CaCO3 deposition will be inhibited. In equation (5), the evaporation of CO2 from the drip water is the rate-limiting step and slows the reaction going toward the right. All the other reactions are much faster.
The importance of the soil carbon dioxide
The drip water calcium ion concentration in the cave depends upon the amount of carbonic acid formed in reactions (1) to (4) in the soil. Then, as the carbonic acid seeps down through cracks in the carbonate, calcium is dissolved by reaction (5) going to the left. The amount of calcium liberated to the seeping water depends upon the amount of soil CO2(aq)—the main variable that controls the growth of speleothems.
Table 2. Five main soil respiration processes

Soil water carbon dioxide is a product of soil respiration when oxygen in the soil is converted to gaseous carbon dioxide and then to soil water carbon dioxide by reaction (1). Much is still unknown about soil respiration.24,25 There are five types of soil respiration: (1) root respiration; (2) rhizomicrobial respiration; (3) decomposition of plant residues; (4) the priming effect induced by root exudation or by addition of plant residues; and (5) the basal respiration by microbial decomposition of soil organic matter (SOM) (table 2).26 Two minor abiotic sources for soil CO2(aq) are dissolution of carbonate in the soil and chemical oxidation,27 which will be ignored. The first four mechanisms are related to the existence of trees and plants, whose soil effects can last several years, and is called autotrophic respiration. The fifth is called heterotrophic respiration. It also depends upon the time of day, the season, the root type, the root size, and the nitrogen content.28 If all vegetation dies out, heterotrophic respiration would continue as long as there is SOM, which can last for centuries before being used up.29 SOM respiration depends upon the quality and quantity of SOM.27 Considering all of the above caveats, estimating the soil CO2(aq) is difficult.
In general, researchers assume that autotrophic respiration accounts for 50% of soil carbon dioxide and heterotrophic respiration 50%. However, the proportion of soil carbon dioxide from autotrophic and heterotrophic respiration varies considerably over the earth, with autotrophic respiration ranging from 10% to 90%.27 To determine the amount of autotrophic respiration, Hogberg et al. girdled (killed) trees and measured the change in soil respiration, which decreased 54% in 1 to 2 months.30
Soil respiration rates depend strongly on many environmental variables: air and soil temperature, soil moisture, evaporation from the soil, seasonality, soil depth, vegetation type and density, type of vegetation cover, and oxygen concentration in the soil air.4,6,16,31 However, the vegetation type apparently does not matter much.32
Soil temperature is the most important factor controlling soil respiration rates. Some researchers believe soil respiration increases exponentially with a rise in temperature.27 However, the situation is much more complicated.33 The exponential increase with rising temperature is probably a short-term result since the SOM decreases, and therefore the effect of a temperature increase in the long term is small.34 Nonetheless, the importance of soil temperature is reflected in the fact that forested tropical soils have the highest soil carbon dioxide.35 Still, other factors contribute, such as that they are thicker, warmer, and form soil carbon dioxide all year around, while at mid and high latitudes soil carbon dioxide decreases in winter because of cooler temperatures and/or frozen ground. This is also the reason why caves in tropical climates have great speleothem growth. The water content of the soil is probably the second most important variable. If it is too dry, soil respiration decreases substantially, but if it is too wet, oxygen cannot be transferred fast enough for soil respiration.20 So, an intermediate amount of soil moisture is ideal.
The importance of vegetation was shown when the area above a mine in Wiltshire, England, was revegetated with a deciduous forest; the calcite deposition rate on a mine stalagmite increased fourfold.36 Under ideal conditions, the partial pressure of soil CO2(aq) can reach an incredible 100,000 ppm or 1/10th of an atmosphere.6,37 However, measured CO2 rarely reaches 100,000 ppm. The total amount of soil carbon dioxide theoretically could reach 210,000 ppm38 if all the soil oxygen were somehow all used up, but this is likely impossible. These figures compare with an atmospheric CO2 partial pressure of 400 ppm. Regardless, soil CO2(aq) is usually about 100 times that of the atmosphere. High soil CO2(aq) results in high Ca(aq)2+ and HCO3(aq)- in the cave drip water, which will result in a rapid growth of cave speleothems.
Flow from soil to cave
Once the water leaves the soil and descends downward through the vadose zone, the carbonic acid dissolves the carbonate according to reaction (5). Dissolution normally occurs within the top 10 m (33 ft) of the epikarst, where the solution equilibrates and no more calcium carbonate is dissolved.39
The amount of Ca2+ entering the water will depend not only upon the amount of carbonic acid in the water, but also whether the vadose zone is a closed or open system. A closed system is one in which the percolating water that seeps down into the vadose zone has little air in the interstices compared to the amount of water.40 An open system is one in which there is abundant air in the vadose zone. In an open system that can have a high amount of CO2 in the air (see below), the amount of CO2 absorbed and carbonic acid formed is significantly greater than in a closed system.41,42 In reality, vadose zone water percolation is usually neither totally closed nor totally open, but somewhere in between.40
Besides the air in vadose zone joints and faults, water can obtain a little more carbonic acid from roots that can sometimes go deep into the epikarst, which also helps with the water flow.43,44 Moreover, organic matter can wash down into the karst to provide more air carbon dioxide for the water.45 In fact, some researchers believe that the main source of the carbon in speleothems comes from SOM washed down into the vadose zone.9 Drilling on the Rock of Gibraltar discovered that many caves and voids were penetrated with high carbon dioxide content.45
As water flows from the soil down into the cave, it can also deposit calcite in air pockets, joints, faults, and cave ceilings, which is called prior calcite precipitation (PCP).46 This can only happen if these air-filled voids are low in CO2, which normally does not occur. Of course, as the water issues from a crack in the ceiling of a cave, it will start depositing calcite on the roof before it reaches the tip of a stalactite. Since researchers are mostly focused on stalagmites, calcite deposition on the stalactite is also usually included in PCP.47 It is also known that the faster the discharge, the less the PCP, and vice versa. So heavy precipitation would favour faster stalagmite growth with less PCP.46
Temperature
Temperature is a significant variable. The warmer the cave temperature, the faster the deposition of CaCO3, especially if winters are mild. Many caves at mid and high latitudes show a significant decrease in the generation of soil CO2(aq) and speleothem growth. The decrease in soil CO2(aq) in winter would be averaged with the summer increase, and such seasonal effects would diminish the average Ca(aq)2+ and HCO3(aq)- in the drip water, and hence slow speleothem growth rate. This however may not have an immediate effect. The drip water moves downward through the limestone at quite variable rates and mixes with water that may be a few years old. Alternatively, the drip water can reach the cave in a matter of days, weeks, or months, depending upon the particular path it takes through the vadose zone.
Areas that became glaciated during the Ice Age would have produced little growth in speleothems during the time of ice coverage. There are a few exceptions, e.g. speleothems can form under glaciers and karst with no soil or vegetation.48,49 Several mechanisms have been suggested for this anomaly.
Since cave temperatures are generally similar to the average surface air temperature, cold winter temperatures will reduce the deposition rate of CaCO3 in the cave during winter. However, in some locations increasing winter ventilation (discussed next) due to storminess could actually cause an increase of CaCO3 deposition, despite cooler winter temperatures. This is the case in central Texas caves where winters are mild and ventilation more than makes up for the cooler temperatures.50
Cave atmospheric partial pressure of CO2
As the drip water exits the carbonate rock above the cave, CO2(aq) leaves the water in the form of a gas, CO2(g).17 The speed of degassing CO2 depends upon the amount of CO2 within the cave atmosphere. The larger the difference between liquid and gaseous carbon dioxide, the faster CO2 in the water degasses and carbonate is added to the speleothem. As long as CO2(aq) is greater than CO2(g) within the cave, calcite deposition will occur. But, as CO2 is added to the cave atmosphere during the above process, the rate of degassing will decrease. CO2 can also be added to a cave if a stream runs through the cave.17 For every molecule of CO2 expelled from the water, one molecule of CaCO3 is deposited either on the cave wall or on speleothems.51 All reactions within the water proceed rapidly except the change of CO2(aq) to CO2(g), which becomes the rate limiting reaction.51 Therefore, speleothems will grow faster with lower partial pressure of CO2 in the cave.52,53
Cave air CO2 today can be quite high and variable, generally from about 800 ppm to over 8,000 ppm,17 which was added to the cave from CO2(aq) changing to CO2(g). Central Texas caves were measured to have a carbon dioxide content as high as 37,000 ppm.54 Therefore, the growth of speleothems will strongly depend upon how well the cave is ventilated (removing excess CO2 gas); the morphology of the cave passages; the size, number, and location of entrances; and the distance from an entrance. All these variables depend especially on the climate.

Cave air is ventilated especially by the surface wind that causes a sucking action. Pressure and temperature differences between the cave and the outside air also will cause air exchange. Even moisture differences can drive a circulation.52,53 As a result, cave ventilation is subject to forcing by diurnal, seasonal, and weather pattern variations. As such, ventilation of most caves is relatively fast.55 Mid- and high-latitude caves commonly have lower cave air carbon dioxide in winter than in summer.52 This is due to increased storminess, wind, and cooler air temperatures than the interior of the cave. Low cave air CO2 levels in winter is one reason why some stalagmites in Texas caves grow faster during the winters than summers.54 Cave CO2 also depends upon the location within the cave, with passageways farther from the exits having more cave air CO2.4 When the entrance is higher than the cave, air convection in the winter causes cold air to sink into the cave and warm air to exit above the entering cold air (figure 3).56 It probably does not take much cave air exchange to keep the cave air CO2 relatively low, as winters in Texas, which are not all that stormy, demonstrate.
Drip water flow rate
It is well known that percolating water from above follows different paths for different stalagmites—there is fast flow through cracks that arrives quickly after precipitation, and there is diffuse flow that may take a year or more. The characteristics of the drip also depend on the thickness of the carbonate above the cave; the porosity and permeability of the vadose zone carbonate; the purity of the bedrock; and the particular path of the groundwater, which can vary with time.4,16 So, each speleothem in each cave is unique with a unique drip rate. Moreover, the flow path can change, which is one reason why the drip rate can vary significantly at any one stalagmite. In general, however, the higher the drip rate, the faster a speleothem will grow,7,57 although some researchers do not find a relationship.58
Cave water drip rate is also non-linearly related to effective precipitation. Effective precipitation is the precipitation minus any soil drying.3,6,36 So, the greater the precipitation and the less the soil evaporation, the greater the drip rate.7 Faster drip rates increase stalagmite radius.59 The growth rate is also related to drop volume, which depends on several variables, such as the radius, surface curvature, and geometry of the stalactite tip.60 Many studies of growth rates use conservative drip volumes.
Thickness of the water film on the stalagmite
The last variable, water film thickness on the stalagmite, is difficult to evaluate. But, the thicker the film, the faster CaCO3 deposition occurs on the stalagmite, which also depends upon the morphology of the apex, and deposition decreases linearly with increasing apex curvature.61 If the apex is nearly flat, the film thickness can be about 1 mm or more, resulting in high growth rate.61 The higher the drop fall, the thinner the film due to splashing, another important variable. However, high drop fall and splash also degasses CO2 faster, which causes more drip water calcium supersaturation that can aid growth.
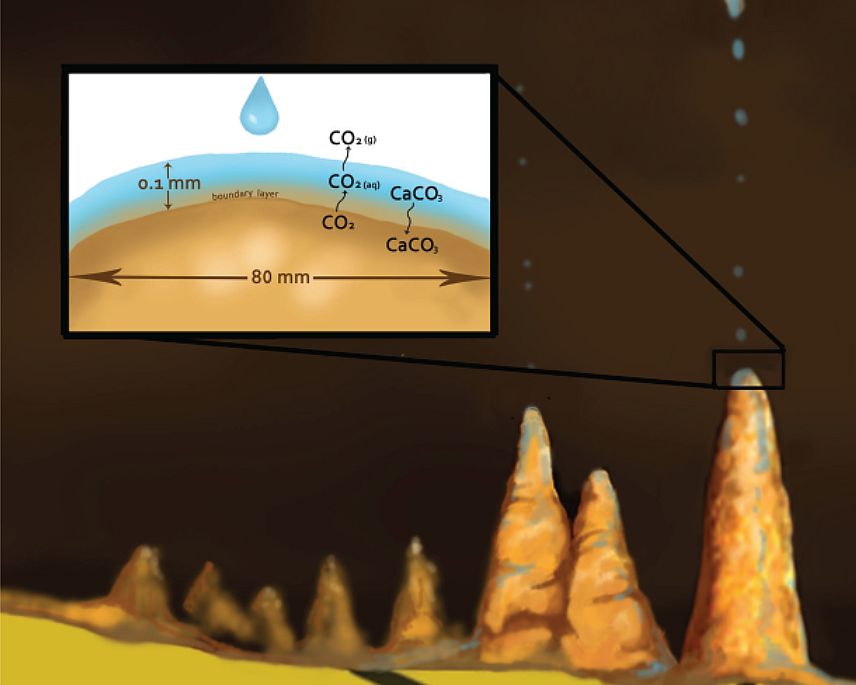
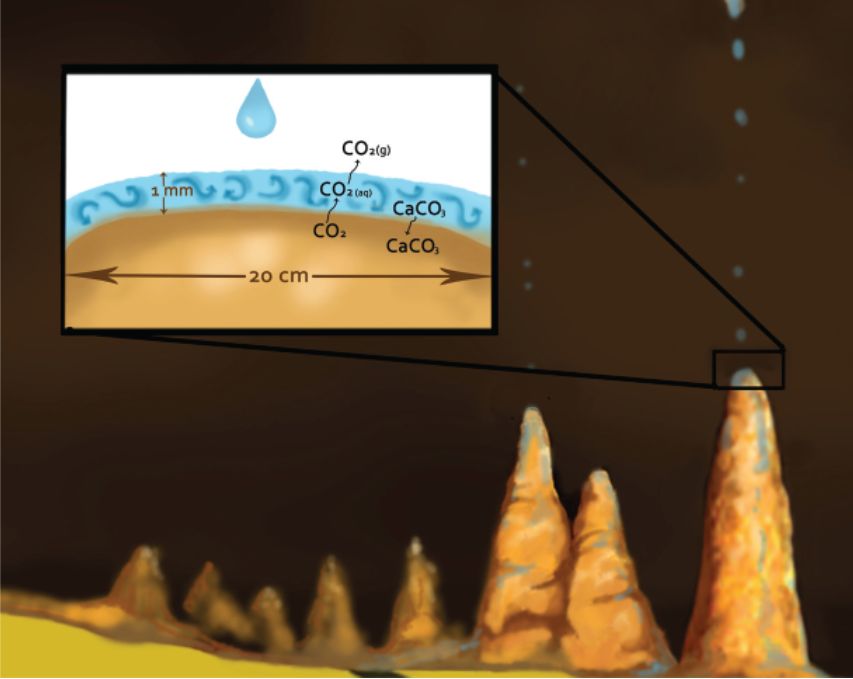
Film thickness is often assumed to be only 0.1 mm since stalagmites are usually convex up.31 But the deposition rate is not as fast as often assumed because of a kinetic effect, which is complicated. In the thin film of water that is either stagnant or moving in laminar flow on top of the stalagmite, there is a very thin boundary layer next to the calcite (figure 4). At the surface of the carbonate, one molecule of calcium carbonate is added, while one molecule of CO2 is liberated,51 which causes concentration gradients within the thin film. The deposition rate depends upon the diffusion of Ca2+ and HCO3- from the layer above through the boundary layer to the surface of the stalagmite. At the same time, CO2(aq) must diffuse from the carbonate surface upward into the layer above, where it degasses. It is degassing of CO2 at the top of the water film that is rate limiting and keeps the process rather slow.4,62 Concentration gradients build up in the thin film that must be relieved by diffusion, a slow process.
The growth will of course depend upon the thickness of the boundary layer. A thick boundary layer will result in slower diffusion. But in turbulent flow, generally considered when the film thickness is 1 mm or greater, the boundary layer is much thinner, so that diffusion length is much less between the thin boundary layer and the thick film above, and so mixing between the layers occurs much faster (figure 5). In this case, deposition is about 10 times as fast.5,63 Moreover, the thicker the film, the more the ions are available above the boundary layer to diffuse downward into the boundary layer. However, turbulent flow is not expected on the top of a stalagmite, at least between drops, but it could occur briefly during splashing of the next drop. Turbulent flow normally occurs when the water is flowing down a slope. This is why flowstone can form much faster than carbonate deposition on stalactites and stalagmites.
Evaporation
There is a sixth variable that could contribute to growth in the evaporation of water from the speleothem but rarely applies in caves today. As equation (5) shows, water loss means that CaCO3 must be deposited. Evaporation is usually ignored since the relative humidity in most caves today is near 100% due to evaporation and underground streams.
However, if ventilation is significant with even a little wind in the cave, and even a slightly lower relative humidity, speleothems can grow.64 At a relative humidity of 88%, 10 times the evaporation occurs than at a relative humidity of 99%.65 The relatively low relative humidity, as well as low CO2, applies to Carlsbad Caverns, which does have good ventilation. However, this is mostly because very little water is dripping into the cave with few speleothems actually growing. Practically all speleothems in Carlsbad Caverns are considered relic, formed in some past climate that is not occurring today.65 For those speleothems that are growing, 40% of the growth rate is attributed to evaporation. Evaporation also strongly affects the oxygen and carbon isotope ratios and the paleoclimate inferences.66
Regardless, if ventilation is strong enough in the past, the reduced relative humidity could be another significant variable for speleothem growth.67
Summary
We have analyzed the five main variables that determine speleothem growth. It is the calcium ion concentration in the drip water that is the most important variable, which strongly depends upon the amount of soil carbon dioxide percolated downward through the vadose zone. This is where the carbonic acid from the soil quickly dissolves some of the CaCO3 until equilibrium, a point reached where it is no longer an acid. But additional changes in the ion concentrations in the drip can occur on the way down to the cave. When the high partial pressure of carbon dioxide enters the cave in the drip water, it is rapidly degassed, inversely proportional to the partial pressure of the carbon dioxide in the cave. In general, speleothem growth is proportional to the temperature of the cave, the amount of drip water falling from the ceiling, and the thickness of the water film. Evaporation can also cause speleothem growth if cave ventilation is significant, such as observed at Carlsbad Caverns. All the relationships between these variables are usually too much to track effectively, so researchers often rely on empirical relationships between major variables, such as the Ca ion concentration and/or the supersaturation, to measure the relative impact of different factors on speleothem growth rates.
In part 3, we will show that these variables, plus variables from the Flood, would maximize speleothem growth in the Ice Age, especially early in the Ice Age.
References and notes
- Liu, A. and Dreybrodt, W., Dissolution kinetics of calcium carbonate minerals in H2O-CO2 solutions in turbulent flow: the role of the diffusion boundary layer and the slow reaction H2O + CO2 ↔H+ + HCO3-, Geochimica et Cosmochimica Acta 61(14):2879–2889, 1997. Return to text.
- Dreybrodt, W., Processes in Karst Systems: Physics, chemistry, and geology, Springer-Verlag, New York, 1988. Return to text.
- Baker, A., Barnes, W.L., and Smart, P.L., Variations in the discharge and organic matter content of stalagmite drip waters in Lower Cave, Bristol, Hydrological Processes 11:1541–1555, 1997. Return to text.
- Baldini, J.U.L., McDermott, F., Hoffmann, D.L., Richards, D.A., and Clipson, N., Very high-frequency and seasonal cave atmosphere pCO2 variability: implications for stalagmite growth and oxygen isotope-based paleoclimate records, Earth and Planetary Science Letters 272:118–129, 2008. Return to text.
- Dreybrodt, W., Chemical kinetics, speleothem growth and climate, Boreas 28:347–356, 1999. Return to text.
- Kaufmann, G., Stalagmite growth and palaeo-climate: the numerical perspective, Earth and Planetary Science Letters 214:251–266, 2003. Return to text.
- Banner, J.L., Guilfoyle, A., James, E.W., Stern, L.A., and Musgrove, M., Seasonal variations in modern speleothem calcite growth in central Texas, USA, J. Sedimentary Research 77:615–622, 2007. Return to text.
- Wong, C.I. and Breecker, D.O., Advances in the use of speleothems as climate archives, Quaternary Science Reviews 127:1–18, 2015. Return to text.
- Noronha, A.L., Johnson, K.R., Southon, J.R., Hu, C., Ruan, J., and McCabe-Glynn, S., Radiocarbon evidence for decomposition of aged organic matter in the vadose zone as the main source of speleothem carbon, Quaternary Science Reviews 127:37–47, 2015. Return to text.
- Belli, R., Borsato, A., Frisia, S., Drysdale, R., Maas, R., and Greig, A., Investigating the hydrological significance of stalagmite geochemistry (Mg, Sr) using Sr isotope and particulate element records across the Late Glacial-to-Holocene transition, Geochimica et Cosmochimica Acta 199:247–263, 2017. Return to text.
- Sinclair, D.J., Banner, J.L., Taylor, F.W., Partin, J., Jenson, J., Mylroie, J., Goddard, E., Guinn, T., Jocson, J., and Miklavic, B., Magnesium and strontium systematics in tropical speleothems from the Western Pacific, Chemical Geology 294–295:1–17, 2012. Return to text.
- Riechelmann, S., Schröder-Ritzrau, A., Wassenburg, J.A., Schreuer, J., Richter, D.K., Richelmann, D.F.C., Terente, M., Constantin, S., Mangini, A., and Immenhauser, A., Physicochemical characteristics of drip water: influence of mineralogy and crystal morphology of recent cave carbonate precipitates, Geochimica et Cosmochimica Acta 145:13–29, 2014. Return to text.
- Wong, C.I. and Breecker, D.O., Advancements in the use of speleothems as climate archives, Quaternary Science Reviews 127:1–18, 2015. Return to text.
- Cheng, H., Sinha, A., Wang, X., Cruz, F.W., and Edwards, R.L., The global paleomonsoon as seen through speleothem records from Asia and the Americas, Climate Dynamics 39:1048, 2012. Return to text.
- Oard, M.J., Rapid growth of caves and speleothems—part 3: flood and Ice Age variables, J. Creation 34(2):75–81, 2020. Return to text.
- Baker, A., Genty, D., Dreybrodt, W., Barnes, W.L., Mockler, N.J., and Grapes, J., Testing theoretically predicted stalagmite growth rate with recent annually laminated samples: implications for past stalagmite deposition, Geochimica et Cosmochimica Acta 62(3):393–404, 1998. Return to text.
- Ek, C. and Gewelt, M., Carbon dioxide in cave atmospheres: new results in Belgium and comparison with some other countries, Earth Surface Processes and Landforms 10:173–187, 1985. Return to text.
- Meyer, K.W., Feng, W., Breecker, D.O., Banner, J.L., and Guilfoyle, A., Interpretation of speleothem calcite δ13C variations: evidence from monitoring soil CO2, drip water, and modern speleothem calcite in central Texas, Geochimica et Cosmochimica Acta 142:281–298, 2014. Return to text.
- Dreybrodt, W., Lauckner, J., Zaihua, L., Svensson, U., and Buhmann, D., The kinetics of the reaction CO2 +H2O → H+ + HCO3- as one of the rate limiting steps for the dissolution of calcite in the system H2O– H2O–CaCO3, Geochimica et Cosmochimica Acta 60(18):3375–3381, 1996. Return to text.
- Fang, C. and Moncrieff, J.B., A model for soil CO2 production and transport 1: model development, Agricultural and Forest Meteorology 95:225–236, 1999. Return to text.
- Hursh, A., Ballantyne, A., Cooper, L., Maneta, M., Kimball, J., and Watts, J., The sensitivity of soil respiration to soil temperature, moisture, and carbon supply at the global scale, Global change biology 23:2090–2103, 2017. Return to text.
- Oard, M.J., Rapid growth of caves and speleothems: part 1—the excavation of the cave, J. Creation 34(1):71–78, 2020. Return to text.
- Zaihua, L., Svensson, U., Dreybrodt, W., Daoxian, Y., and Buhmann, D., Hydrodynamic controls of inorganic calcite precipitation in Huanglong Ravine, China: field measurements and theoretical predictions of deposition rates, Geochimica et Cosmochimica Acta 59(15):3087–3097, 1995. Return to text.
- Davidson, E.A., Janssens, I.A., and Luo, Y., On the variability of respiration in terrestrial ecosystems: moving beyond Q10, Global Change Biology 12:154–164, 2006. Return to text.
- Bond-Lamberty, B. and Thompson, A., Temperature-associated increases in the global soil respiration record, Nature 464:479–582, 2010. Return to text.
- Kuzyakov, Y., Sources of CO2 efflux from soil and review of partitioning methods, Soil Biology and Biochemistry 38:425–448, 2006. Return to text.
- Pumpanen, J., Ilvesniemi, H., and Hari, P., A process-based model for predicting soil carbon dioxide efflux and concentration, Soil Science Society of America J. 67:402–413, 2003. Return to text.
- Trumbore, S., Carbon respired by terrestrial ecosystems—recent progress and challenges, Global Change Biology 12:141–153, 2006. Return to text.
- Noronha, A.L., Johnson, K.R., South, J.R., Hu, C., Ruan, J., and McCabe-Glynn, S., Radiocarbon evidence for decomposition of aged organic matter in the vadose zone as the main source of speleothem carbon, Quaternary Science Reviews 127:37–47, 2015. Return to text.
- Högberg, P., Nordgren, A., Buchmann, N., Taylor, A.F.S., Ekblad, A., Högberg, M.N., Nyberg, G., Ottosson-Löfvenius, M., and Read, D.J., Large-scale forest girdling shows that current photosynthesis drives soil respiration, Nature 411:789–792, 2001. Return to text.
- Kaufmann, G. and Dreybrodt, W., Stalagmite growth and palaeo-climate: an inverse approach, Earth and Planetary Science Letters 224:529–545, 2004. Return to text.
- Raich, J.W. and Tukekcioglu, A., Vegetation and soil respiration: correlations and controls, Biogeochemistry 48:71–90, 2000. Return to text.
- Conant, R.T., et al., Temperature and soil organic matter decomposition rates—synthesis of current knowledge and a way forward, Global Change Biology 17:3392–3404, 2011. Return to text.
- Karhu, K., et al., Temperature sensitivity of soil respiration rates enhanced by microbial community response, Nature 513:81–84, 2014. Return to text.
- Amundson, R.G. and Davidson, E.A., Carbon dioxide and nitrogenous gases in the soil atmosphere, J. Geochemical Exploration 38:13–41, 1990. Return to text.
- Baldini, J.U.L., McDermott, F., Baker, A., Baldini, L.M., Mattey, D.P., and Railsback, L.B., Biomass effects on stalagmite growth and isotope ratios: a 20th century analogue from Wiltshire, England, Earth and Planetary Science Letters 240:486–494, 2005. Return to text.
- Austin, A.A., Origin of limestone caves, Acts & Facts Impact No. 79, Institute for Creation Research, Dallas, TX, 1980. Return to text.
- Brook, G.A., Folkoff, M.E., and Box, E.O., A world model of soil carbon dioxide, Earth Surface Processes and Landforms 8:79–88, 1983. Return to text.
- Silvestru, E., Caves for all seasons, Creation 25(3):44–49, 2003. Return to text.
- Drake, J.J., The effects of geomorphology and seasonality on the chemistry of carbonate groundwater, J. Hydrology 61:223–236, 1983. Return to text.
- Buhmann, D. and Dreybrodt, W., The kinetics of calcite dissolution and precipitation in geologically relevant situations of karst areas: 1. open system, Chemical Geology 48:189–211, 1985. Return to text.
- Buhmann, D. and Dreybrodt, W., The kinetics of calcite dissolution and precipitation in geologically relevant situations of karst areas: 2. closed system, Chemical Geology 53:109–124, 1985. Return to text.
- Breecker, D.O., Payne, A.E., Quade, J., Banner, J.L., Ball, C.E., Meyer, K.W., and Cowan, B.D., The sources and sinks of CO2 in caves under mixed woodland and grassland vegetation, Geochimica et Cosmochimica Acta 96:230–246, 2012. Return to text.
- Treble, P.C., Fairchild, I.J., Baker, A., Meredith, K.T., Andersen, M.S., Salmon, S.U., Bradley, C., Wynn, P.M., Hankin, S.I., Wood, A., and McGuire, E., Roles of forest bioproductivity, transpiration and fire in a nine-year record of cave dripwater chemistry from southwest Australia, Geochimica et Cosmochimica Acta 184:132–150, 2016. Return to text.
- Mattey, D.P., Atkinson, T.C., Barker, J.A., Fisher, R., Latin, J.-P., Durrell, R., and Ainsworth, M., Carbon dioxide, ground air and carbon cycling in Gibraltar karst, Geochimica et Cosmochimica Acta 184:88–113, 2016. Return to text.
- Borsato, A., Johnston, V.E., Frisia, S., Miorandi, R., and Corradini, F., Temperature and altitudinal influence on karst dripwater chemistry: implications for regional-scale palaeoclimate reconstructions from speleothems, Geochimica et Cosmochimica Acta 177:275–297, 2016. Return to text.
- Sherwin, C.M. and Baldini, J.U.L., Cave air and hydrological controls on prior calcite precipitation and stalagmite growth rates: implications for palaeoclimate reconstruction using speleothems, Geochimica et Cosmochimica Acta 75:3915–3929, 2011. Return to text.
- Dreybrodt, W., A possible mechanism for growth of calcite speleothems without participation of biogenic carbon dioxide, Earth and Planetary Science Letters 58:293–299, 1982. Return to text.
- Atkinson, T.C., Growth mechanisms of speleothems in Castleguard Cave, Columbia Icefields, Alberta, Canada, Arctic and Alpine Research 15(4):523–536, 1983. Return to text.
- Musgrove, M., Banner, J.L., Mack, L.E., Combs, D.M., James, E.W., Cheng, H., and Edwards, R.L., Geochronology of late Pleistocene to Holocene speleothems from central Texas: implications for regional paleoclimate, GSA Bulletin 113(12):1532–1543, 2001. Return to text.
- Dreybrodt, W., Eisenlohr, L., Madry, B., and Ringer, S., Precipitation kinetics of calcite in the system H+ + HCO3- → CO2 +H2O as a rate limiting step, Geochimica et Cosmochimica Acta 61(18):3897–2904, 1997. Return to text.
- Buecher, R.H., Microclimate study of Kartchner Caverns, Arizona, J. Cave and Karst Studies 61(2):108–120, 1999. Return to text.
- Spötl, C., Fairchild, I.J., and Tooth, A.F., Cave air control on dripwater geochemistry, Obir Cave (Austria): implications for speleothem deposition in dynamically ventilated caves, Geochimica et Cosmochimica Acta 69(10):2451–2468, 2005. Return to text.
- Wong, C.I., Banner, J.L., and Musgrove, M., Seasonal dripwater Mg/Ca and Sr/Ca variations driven by cave ventilation: implications for and modeling of speleothem paleoclimate records, Geochimica et Cosmochimica Acta 75:3514–3529, 2011. Return to text.
- Kowalczk, A.J. and Froelich, P.N., Cave air ventilation and CO2 outgassing by radon-222 modeling: how fast do caves breathe? Earth and Planetary Science Letters 289:209–219, 2010. Return to text.
- Fernandez-Cortes, A., Calaforra, J.M., and Sanchez-Martos, F., Spatiotemporal analysis of air conditions as a tool for the environmental management of a show cave (Cueva del Agua, Spain), Atmospheric Environment 40:7378–7394, 2006. Return to text.
- Dreybrodt, W., Deposition of calcite from thin films of natural calcareous solutions and the growth of speleothems, Chemical Geology 29:89–105, 1980. Return to text.
- Genty, D., Baker, A., and Vokal, B., Intra- and inter-annual growth rate of modern stalagmites, Chemical Geology 176:191–212, 2001. Return to text.
- Romanov, D., Kaufmann, G., and Dreybrodt, W., Modeling stalagmite growth by first principles of chemistry and physics of calcite precipitation, Geochimica et Cosmochimica Acta 72:423–437, 2008. Return to text.
- Collister, C. and Mattey, D., Controls on water drop volume at speleothem drip sites: an experimental study, J. Hydrology 358:259–267, 2008. Return to text.
- Baker, A.J., Mattey, C.P., and Baldini, J.U.L., Reconstructing modern stalagmite growth from cave monitoring, local meteorology, and experimental measurements of dripwater films, Earth and Planetary Science Letters 392:239–249, 2014. Return to text.
- Dreybrodt, W. and Deininger, M., The impact of evaporation to the isotope composition of DIC in calcite precipitating water films in equilibrium and kinetic fractionation models, Geochimica et Cosmochimica Acta 125:433–439, 2014. Return to text.
- Dreybrodt, W. and Buhmann, D., A mass transfer model for dissolution and precipitation of calcite from solutions in turbulent motion, Chemical Geology 90:107–122, 1991. Return to text.
- Deininger, M., Fohlmeister, J., Scholz, D., and Mangini, A., Isotope disequilibrium effects: the influence of evaporation and ventilation effects on the carbon and oxygen isotope composition of speleothems—a model approach, Geochimica et Cosmochimica Acta 96:57–79, 2012. Return to text.
- Hill, C.A., Geology of Carlsbad Cavern and other caves in the Guadalupe Mountains, New Mexico and Texas, New Mexico Bureau of Mines and Mineral Resources Bulletin 117, New Mexico Institute of Mining and Technology, Socorro, NM, 1987. Return to text.
- Mickler, P.J., Stern, L.A., and Banner, J.L., Large kinetic isotope effects in modern speleothems, GSA Bulletin 118(1/2):65–81, 2006. Return to text.
- Hill, ref. 65, p. 115. Return to text.







Readers’ comments
Comments are automatically closed 14 days after publication.