Journal of Creation 36(2):81–89, August 2022
Browse our latest digital issue Subscribe
Racemization of amino acids under natural conditions: part 3—condensation to form oligopeptides
The condensation reaction peptiden + amino acid → peptiden+1 in water is thermodynamically unfavourable by about 2.3 kcal/mol per peptide bond at ~ 30°C and near neutral pH for glycine, due to the stability of zwitterions. An equilibrium ratio of [peptiden+1]/[peptiden] of ~ 1/50 results. For [alanyl-glycine]/[alanine + glycine] the proportion is only about 10–5 under those conditions. Accelerated peptide growth by reactions such as 2 peptiden → peptide2n can only occur in highly concentrated laboratory conditions. Chemically esterifying end carboxyl groups to activate them, sonication, ideal stoichiometries, pH, temperature and pressure are examples of intelligent intervention to force the desired outcome.
Examining reaction parameters systematically (initial concentration of glycine, dehydration times, number of dehydration cycles, temperature, pH, and concentration of NaCl) produced as largest peptide Gly12 in aqueous solution and Gly14 embedded in an insoluble solid. Mass spectrometry analysis suggested Gly20 formed in trace amounts. Products had to be quickly removed after being formed to prevent chemical degradation since amino acids are irreversibly destroyed at ~ 240°C. These experiments demonstrate peptides of relevant size for origin-of-life speculations cannot have arisen naturalistically.
Conditions for peptide condensation reactions
Living organisms depend on proteins to carry out a multitude of functions. These must be composed of L-enantiomer amino acid residues (L-peptiden) to produce reliable secondary structures such as α-helices, β-sheets, and turns which are necessary for stable folding into tertiary structures. Enantiopure amino acids (AAs) will racemize, whether in free form or chemically bound, as we discussed in part 2 of this series.1 Much effort has been expended by the pro-evolution research community on a second problem—namely, to find naturalistic conditions to form long peptides. We will review some key reports on this topic, considering their plausibility for origin of life (OoL) purposes.
L-peptiden must be large and homochiral and both requirements must be satisfied concurrently if an OoL experiment is to have any significance. In part 4 of this series, we will show that optimizing the conditions to obtain larger peptides inevitably racemizes the peptides faster. This fact is hidden by using glycine in the publications, the only proteinogenic AA lacking D and L enantiomers, and thereby incapable of producing folded proteins.
In part 4 we will compare kinetic rate constants and Gibbs free energy changes for racemization and condensation in water. To facilitate this analysis, let us review peptide condensation.
Naturalistic models to drive amino acid condensation
We will not attempt here a detailed review of all the efforts to overcome the unfavourable thermodynamics and kinetics to form polypeptides in aqueous solution under allegedly plausible naturalistic conditions. Without the use of specialized catalysts, very low yields (typically < 1%) of oligomeric products having n > 3 residues have been reported when attempting peptide synthesis on clays,2 minerals,3 at air-water interfaces,4 on metal oxide surfaces,5 and under hydrothermal conditions.6,7 Inevitably only glycine was used, intelligently organized to optimize the intended outcome with no relevance to how unguided processes function in nature. We decided not to analyze those studies which are not realistically amenable to any abiogenesis model.
The overall free energy change of hydrolyzation is unfavourable
For n residues linked in a peptide, there are n – 1 peptide bonds. The free energy of peptide bond hydrolysis vs formation in aqueous solution strongly favours the dissociated, non-condensed products. This is because at pH around 4.5 < pH < 7.5, AAs and peptide fragments form ions which are very stable in water. Therefore, a high free energy is required to generate the neutral form of the reactants necessary to undergo condensation reactions.
To study equilibrium-rate constants and Gibbs free energy, one can begin from either side of the reaction (figure 1). The reverse of condensation, hydrolysis of a peptide bond to neutral products, is unfavourable energetically, contra what most believe; but when it occurs, both fragments generated can then be transformed to the stable aqueous ions.8 This is what makes hydrolysis favourable overall (figure 1). This insight is symmetrical. The main reason that the formation of AA condensation products in water is unfavourable thermodynamically is the stability of the initial zwitterions in water.

The overall free energy change of hydrolyzation, ΔGh, thus includes two effects:
ΔGh = ΔGm + ΔGi [1],
where ΔGm is due to hydrolysis of the amide bond to uncharged products and ΔGi is the free energy of their ionization.8
Martin from the University of Virginia calculated the overall ΔGh of hydrolysis for glycine (G),8 such as via GG ⇌ G + G; GGGG ⇌ GG + GG; and poly-Gn ⇌ poly-Gn–1 + G (table 1).8 G + G ⇌ GG was energetically least favourable with an equilibrium constant Kh ≈ 1/400; for polyGn–1 + G ⇌ polyGn, Kh ≈ 1/50; and GG + GG ⇌ GGGG Kh ≈ 1/10. (The values in the last column of table 1 were rounded off, as the authors of ref. 8 also did.)

ΔGh is the overall free energy of amide hydrolysis, including the ionization step.
ΔGm is the free energy of hydrolysis of the amide bond to uncharged products.
ΔGi is the free energy of ionization.
b Experimental value. All other values in the last two columns were calculated by the author.
c Using the relationship –ΔGh = 2.3RT x log Kh ≈ 1.4 log Kh kcal/mole.
At equilibrium in aqueous solution, ambient temperatures, and a reference concentration of [G] = 1 M, [polyGn] / [polyGn–1] will form at a decreasing proportion of ~ 1/50 per residue added
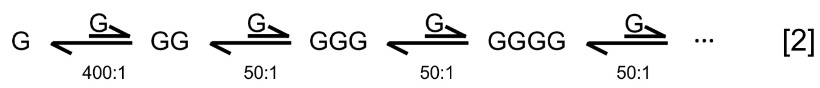
Therefore, the proportion of polyGn to G for n residues at equilibrium is approximately
[polyGn] / [G] = 1/400 × (1/50)n–2 [3]
A small n = 101 residue Gly101 would only be present in a molar ratio to free glycine of < 10–170 at equilibrium at ~ 25–37°C (1/400 × (1/50)99). The general conclusions from table 1 should apply to other AAs. Hydrolysis should be even more favourable due to steric interference for non-Gly residues. Conversely, condensation will be more difficult for AAs containing a bulkier side chain R than H, as found in glycine. Thaxton, Bradley, and Olsen calculated an average free energy of –3.0 kcal/mol per bond.9
Having started with only monomeric G, GG must form before GGG can be produced, and so on for larger peptides. Since the hydrolysis reaction polyGn → polyGn–1 + G is always strongly favoured, and the peptides would be in extremely low concentrations in a plausible abiotic environment, a prohibitively long amount of time would be needed to form condensation product polyGn+1, which, even at equilibrium, would only have a concentration ~ 1/50[polyGn]. Therefore, we neglected possibilities like reaction [4], which would require two molecules both of extreme low concentration to encounter each other and condense, even though thermodynamically less prohibitive than G + GG → GGG.
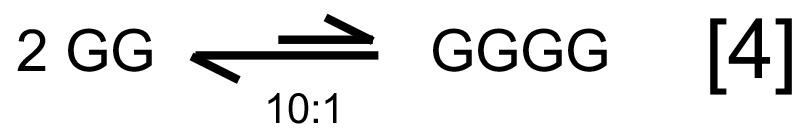
Bada estimated the concentration of amino acids in a putative abiotic ocean to be ~ 10–10 M,10 so at equilibrium a dimer would only have been ~ 1/400 × 10–10 M. Two dimers would therefore be exceedingly unlikely to encounter each other to form GGGG.
Although true that the free energy necessary to condense two peptides is not as high as for two free amino acids, they would be too dilute for this to have been the primary path to form long peptide chains. For abiogenesis purposes, a large environment such as an open ocean is needed to provide the quantity of AAs necessary for a reasonable number of larger peptides to be generated. However, any small peptide would diffuse and be present in very dilute concentrations, so we conclude that k[GG][G] > k’[GG][GG], especially for peptides larger than GG.
Brack reported even less attractive numbers for condensations of other AAs. The free energy for the condensation of alanine and glycine to form alanyl-glycine in H2O was calculated to be + 4.13 kcal/mol at 37°C and pH 7. The equilibrium concentration of the dipeptide for 1 M solutions of the free AA is only slightly above 10–5 M.11
Germane to part 4, immense amounts of time would be needed to build up peptides naturally and during the entire time the amino acids would be racemizing.
Oligomerization under high temperature and pressure
Since larger peptides don’t form in water naturally, considerable effort has been dedicated to finding suitable conditions to make this possible. Matsuno, at the Nagaoka University of Technology, designed a flow reactor intended to simulate a submarine hydrothermal environment, although he acknowledges some large differences; for instance, in pH, CO2, Na, and Cl contents.12 In one series of experiments, 100 mM solutions of glycine dissolved in pure water were introduced into a high-temperature chamber set at 24.0 MPa pressure, which is slightly above the pressure of the critical point of water, thus keeping it liquefied. The effect of temperature was examined between 110° and 350°C in different runs, with 200°–250°C giving the best condensation results.
After a few seconds in the hot chamber, liquid was forced out into a cold chamber containing water also at 24.0 MPa near 4°C to prevent dissociation reactions such as decarboxylation, deamination, and dehydration. The fluid flowing out of the low-temperature chamber was first depressurized to normal atmospheric pressure, and 5 μl aliquots were examined with HPLC. High pressure was reapplied to force the flow back into the original hot chamber. In this manner solutions producing oligopeptides were repeatedly reintroduced as reactants, using very short cycle times of 34 and 78 s. Outside such a laboratory setup any oligopeptides formed would diffuse into the huge ocean volume instead, remaining in place and permitting further condensation. After about ten minutes the yields no longer increased for the desired products: diketopiperazine (a cyclic peptide formed by dimerizing two glycines) (> 1% yield), the glycine dimer (~ 1%) and glycine trimer (<1%). No larger peptides were found. The absence of tetraglycine suggests that it was rapidly hydrolyzed into two molecules of diglycine. These were decidedly unspectacular results.
In a second set of experiments, 10 mM CuCl2 was added to the 100 mM glycine solution at a pH of 2.5 (to facilitate condensation), at 250°C and 24.0 MPa in the hot chamber. The cycle time was maintained at 34 s. Now four different oligomers were found, with the following yields after 30 minutes: diketopiperazine (~ 0.1%), (Gly)2 (< 0.01%), (Gly)4 (~ 0.1%), and (Gly)6 (~ 0.001%).
The copper ions seem to have prevented the hydrolysis of tetraglycine. The authors interpreted the presence of even-numbered oligopeptides up to hexaglycine and the absence of both tri- and pentaglycine to mean that the chain elongation proceeds mainly by aminolysis of diketopiperazine.
The experiments were cleverly terminated after such a short time to prevent degradation of the products. The results confirm the work conducted by Bada in 1995 to evaluate hydrothermal vent type chemistry, who concluded that amino acids are irreversibly destroyed by heating at ~ 240°C.13
In later work, reported in 2000, Matsuno et al. worked with 40 mL L-alanine, which has no reactive side groups, at 250°C and identified (Ala)2 (< 1%), (Ala)3 (< 0.01%) and (Ala)4 (~ 0.001%). In the same paper, they optimized the conditions and re-examined 100 mM glycine mixed with 10 mM CuCl2 for a longer experimental time (2 hrs) and managed to produce (Gly)8 in ~ 0.001% yield.14 The low yield reflects decomposition due to longer exposure to high temperature.14
These experiments demonstrate that hydrothermal vent-type environments are not plausible environments for OoL models. The carefully optimized experimental conditions included high concentrations of initial AA at a boundary between ~ 250°C and icy water; recirculation of peptides at an optimal rate to concentrate them in a high-pressure, low-volume region against a high temperature and pressure gradient. Even then it was only possible to generate (Ala)4 and (Gly)8 at concentrations of ~ 0.001% after about two hours (after which they rapidly decomposed thermally).
But this work is valuable for our purposes. It illustrates a point we will emphasize in part 4. The environmental parameters modified to accelerate condensation will also accelerate racemization. In this case, a large amount of Cu2+ was indispensable to obtain some larger oligomers, but the cation would also accelerate the rate of AA racemization dramatically.1,15 In addition, we want to compare racemization and condensation rates at all temperatures, and Matsuno et al. have given us important empirical data for how long it would take to form small peptides at the high temperature of 250°C in water under pressure. We will use the Arrhenius equation in part 4 to show that L → D conversion under these conditions would be several orders of magnitude faster than condensation.
Scenarios to minimize hydrolysis of peptides
Special environmental conditions have been proposed to counteract the unfavourable AA condensation thermodynamic and kinetic effects. One environment involves thin hydrophobic air-water interfaces such as those potentially found at the surfaces of lakes and oceans, and in atmospheric aerosols.16 Griffith and Vaida conducted some experiments using a Langmuir-trough and chemically activated AA leucine having the end carboxyl group converted to ethyl ester.16 Here we encounter the familiar intelligent intervention by OoL scientists necessary to force the desired outcome. AA-esters condense considerably more readily than normal untransformed AAs. In addition, for the condensation to proceed, coordination to Cu2+ (copper (II) chloride) was used, and an external pressure of 15 mN/m was also necessary, using the mechanical barriers of the Langmuir trough. The final solution was sonicated until a transparent solution resulted.16
The surface pressure of 15 mN/m was chosen to orient the surface molecules, facilitating complexing with Cu2+ and hindering condensation products from diffusing into the bulk solution. Mechanistically, the Cu2+ probably coordinates to the NH2 of both AAs. Without compression, the adsorbed molecules did not form complexes with the copper ions or react in any way.16 The authors did not comment on how this affected the relevance to natural conditions.
The condensation was set up to proceed overnight, but the yield of dipeptide was not reported. Whether further subsequent condensation of an AA to a peptide occurs under these conditions was not reported.
We included this example as a representative study, often cited as evidence, which used conditions known to facilitate peptide condensation. Although an interesting experiment, its quantitative value is highly questionable. Unless one reads the paper carefully, most would not realize, from the references to this work, that instead it reflects the enormous intellectual effort necessary to obtain even the most modest results with no link to natural conditions.
To be relevant to an abiogenesis scenario, one must assume there could have been a constant supply of esterized AAs delivered to this location. No effort was invested to extrapolate from the ideal conditions, which included esterized AAs, ideal surface pressure applied in the correct direction only, sonication, and suitable stoichiometries. The time to build up a relevant concentration of dipeptides and then larger peptides would have been immense, and during this entire time the residues would have been racemizing. Incidentally, we suspect that coordination of Cu2+ would have rendered the two amino groups partially positively charged, facilitating formation of the carbanion intermediate and thereby racemization. More Cu2+ would facilitate dipeptide formation, but also racemization.
Largest oligomers produced without catalysts
Given the very low yields of oligomeric products reported in OoL studies, in spite of highly contrived conditions as mentioned above, Cronin and colleagues at the University of Glasgow developed a digitally controlled reactor system they called the ‘abiotic peptide synthesiser’ to run many experiments in parallel to find the best parameters possible.17 This is valuable work and something we long hoped to do ourselves, demonstrating the best outcome possible under naturalistic conditions, assuming blind chance were to have found these best conditions. Parameters tested included initial concentration of glycine (G, 10–4 M – 10–1 M), dehydration times (1–96 h), number of dehydration cycles (1–4), temperature (90–130°C), pH (2.15–10), and concentration of NaCl (0–1 M).17
The optimal conditions involved injecting an aqueous solution of glycine (0.09 M) containing NaCl (0.25 M), pH ~ 10 adjusted with NaOH, into a preheated vial (130°C), which was then maintained at that temperature for 15 h, evaporating the solution to dryness (the ‘dehydration step’). After only one dehydration-hydration cycle, they observed oligomers in solution with sizes up to Gly12.
The pH had a significant influence. For pH 3.5–7.5 at 130°C, the G → oligomer yield was only ~ 1% of that found at pH ~ 10.18 Unfortunately, the authors did not report what the largest oligomer would be at an OoL realistic pH near neutral. At neutral pH, glycine monomers are zwitterionic, and interactions between the charged amino group and a charged carboxyl group render them ineffective for dimerization. As pointed out above, zwitterions are thermodynamically very stable in water, hindering the condensation reaction.
Raising the temperature from 90°C to 130°C increased the oligomer yield significantly. At 130°C the highest yield (~ 45% at pH 9.75) was achieved in 15 h. Then the yield decreased steadily with time and a brown colour developed (degradation to other substances19), as shown in figure 2. At the intermediate temperature of 110°C, the highest yield was reached at ~ 70 h, then decreased also with time. At the lower temperature of 90°C, the monomer → oligomer yield only reached about 20% after 100 h, as shown in figure 2.20


Oligomer proportion was highest for NaCl = 0.25 M, but the decrease in oligomer yield at higher and lower NaCl was not dramatic.
More than 4 cycles would not be expected to create higher amounts of the larger oligomers, Table 2 and Figure 3.21 With each repeated cycle, decomposition of especially the larger oligomers increases. Concentrations of oligomers ≥ (Gly)14 could not be detected by IP-HPLC (ion pair high-performance liquid chromatography).

Larger oligomers like these (13 residues) are only formed when the initial concentration of glycine is very high (and temperature ~ 100°C above room temperature). To illustrate, concentrations of glycine ranging from 10–4 to 10–1 M were prepared at 130°C. The concentration of the (Gly)3 trimer decreased by almost 3 orders of magnitude when the initial concentration of glycine was decreased from 10–1 M to 10–4 M, as shown in figure 4B.22
A mass spectrometric (MALDI-TOF) analysis revealed a peak at 1181.4, which might be the sodium adduct of (Gly)20, the largest oligomer claimed, but at an unmeasurably low concentration.
No attempts were reported to find the largest oligopeptides when different AAs were mixed with glycine. Many AA side chains can react, and indeed both branched structures and linear peptides were found for the four-residue products analyzed.

Interpreting the optimized condensation reactions correctly
All chemists are trained in the same basic principles, and most of us researching in universities and industrial labs have no idea which of our colleagues believe in evolution or creation, since this does not affect our work practices. Most creation-oriented chemists perform the same kinds of systematic experiments Cronin et al. did.17 We often face the challenge of finding optimal manufacturing conditions to produce the highest yield of a target material. Automated equipment to explore parameter space is best practice at big chemical firms. Cronin has done what we always hoped to do someday: find the largest peptides and their concentrations under the best naturalistic conditions possible. We would have favoured a DoE (Design of Experiment) approach, since it is possible that some combination of intermediate values for number of cycles, cycle duration, pH, temperature, and initial concentration of glycine might produce unexpectedly better results. We would have automated the various tests also.23 Perhaps we could collaborate with the University of Glasgow in an updated project.24
But if this had been our project, we would have written a very different paper or research report. We will show that our conclusions are legitimate, having nothing to do with our position on creation vs evolution. We have done operational science for decades and are concerned that Cronin’s wish to support evolution has prevented the desired objectivity in communicating his results. The authors are justifiably proud of their results and emphasize that at the best pH, temperature, and dehydration duration, a 0.09 M aqueous solution of glycine monomers produced larger oligomers than others have reported (≤ 7), and in only one cycle. That certainly reflects good laboratory work on their part, and we congratulate them.
But the authors fail to state the obvious. They have intelligently explored all parameters and identified the settings producing the largest Glyn, knowing this would not occur naturalistically. Gly20 may have been obtained, but in too low a concentration to measure. An average-size protein has about 300 residues. Furthermore, they only mention in the supplementary materials that the larger peptides are only sparingly soluble, and the HPLC traces revealed no presence of oligomers of size n ≥ 12. The solid precipitates which formed had to be dissolved by adding trifluoroacetic acid. As a tarry amorphous material, such peptides would continue to racemize (even fossilized peptides racemize); therefore, they would serve no purpose for abiogenesis speculations.
Since the project was designed for OoL purposes, they should now clearly state that without researcher interference the natural outcomes could only have been far more modest. The question is how much more. That analysis is missing, and this is exactly why we have wanted to perform similar experiments for years; to extrapolate from theoretical best case towards realistic conditions.
From figure 3 we see that the concentration of peptides decreases rapidly with size. Bada, a leading evolutionist specialist in abiogenesis and amino acids, estimated a concentration of ~ 10–10 M would have been available in putative ancient oceans, and not ~ 0.1 M.10 Cronin et al. showed that decreasing the concentration of initial glycine by a factor of 103 lowered the concentration of Gly2 and Gly3 by a factor of 185 and 583, respectively (figure 4). Not only would this decrease drastically the concentration of the larger peptides over-proportionally, but the diluted dimers and trimers would now rarely come into contact, which was a major reason that larger peptides formed in these experiments. Instead of two now extremely dilute peptides condensing, the polymerization rate becomes almost entirely limited to a peptide having to encounter an amino acid, leading to a peptide only one residue larger. We see once again that deep time is evolutionists’ great enemy, as the now much longer times involved provide opportunities for decomposition by exposure to heat and photolysis.
Isolation of water in a hot environment followed by evaporation would increase the density of AAs from an initial ~ 10–10 M, but also the concentration of many interfering substances, including those mentioned on part 2 which accelerate racemization. The amino acids could not have been delivered from the sources of heat (volcanos, fierce greenhouse effect, meteorite crashes, or hydrothermal vents), since they would have decomposed. Normal lake shore evaporation would be nowhere near 130°C, and condensation between solidified salts won’t occur. A theoretical OoL scenario is not clear. Perhaps the AAs could be delivered by colder water, in which case, after some evaporation, the AAs and peptides would be sure to be diluted again later. But the alternative would be to have them trapped in a hot environment where chemical decomposition becomes inevitable in a matter of hours.
Let us continue modifying the parameters slightly in the direction of more realistic conditions. We will allow for a very heavy dose of optimism in all the parameters, but not to the point of requiring a miracle. The ideal highly basic pH contributed a factor of 10 to 100 improvement. Fifteen hours gave the right results at 130°C. Fifteen hours, not days, millennia, or million years, during which the larger peptides would have completely decomposed. Nature does not work in the manner an astute chemist does. How likely is it that somewhere at around 130°C a collection of concentrated AAs with no interfering substance remained in the right location just long enough before fleeing to safety? Fifteen seconds or minutes would have produced no measurable quantity of large peptides. Everything had to be precisely fine-tuned to obtain good results.
It is common practice to develop mathematical models to perform these kinds of what-if analysis (which is why we would have carried out a DoE first to have the necessary data to develop robust equations). As an example of modelling, one could use reported data to predict the concentration of Gly14 to Gly20 under specific conditions, as shown in figure 5. (Log transformations, as used in figure 5A, are performed to produce reasonably constant prediction errors over wide ranges of y-values). Since the larger peptides are of major interest the concentrations should be measured multiple times, since small measurement errors would hinder good extrapolations.

We summarize in table 3 some straightforward insights gained from the data provided.17 Unfortunately, the reader’s attention was not drawn to these.
Cronin’s pro-evolution bias also seems to be reflected in the fact that several experiments were not conducted which would have been expected from these kinds of agenda-free optimization projects. Conducting such experiments and commenting on them would have offset the distortions perhaps unintentionally provided through the publication.17 For example, in the supplementary materials, figure 16, an IP-HPLC trace is shown for oligomerization products formed at 130°C and 24 h. The baseline is flat where Gly12 and larger peptides were to be found. How reliable are the reported concentrations? But importantly, experiments were run only at the improbable temperatures of 90–130°C.


Why wasn’t a single example run at ~ 50°C, with all other parameters set at the optimal values and then the corresponding IP-HPLC shown in order see what size peptides could be obtained, to provide the reader with some perspective to realistic natural outcomes? Seeing a flat baseline starting around perhaps > Gly8, despite all the other unrealistic parameter settings (glycine 108 times higher than plausible, etc.), would present a correct but, for OoL purposes, inimical picture. Table 4 summarizes some of the tests we suggest be performed and evaluated publicly.
We are grateful for Cronin’s quantitative work, which confirms that the theoretically largest peptides which could form (~ 20 residues in the case of glycine) would only be in trace concentrations after optimizing every environmental parameter. Peptides formed of size ≥ Gly13 would be insoluble. This places severe constraints on the sizes and concentrations of peptides able to form under plausible natural environments and a basis for fruitful discussion on OoL speculations.
References and notes
- Truman, R., Racemization of amino acids under Natural Conditions: part 2—Kinetic and thermodynamic data, J. Creation 36(2):72–80, 2022. Return to text.
- Lahav, N., White, D., and Chang, S., Peptide formation in the prebiotic era: thermal condensation of glycine in fluctuating clay environments, Science 201:67–69, 1978. Return to text.
- Georgelin, T., Jaber, M., Bazzi, H., and Lambert, J.F., Formation of activated biomolecules by condensation on mineral surfaces—a comparison of peptide bond formation and phosphate condensation, Orig. Life Evol. Biosph. 43:429–443, 2013. Return to text.
- Griffith, E.C. and Vaida, V., In situ observation of peptide bond formation at the water-air interface, PNAS 109:15697–15701, 2012. Return to text.
- Shanker, U., Bhushan, B., and Bhattacharjee, G.K., Oligomerization of glycine and alanine catalyzed by iron oxides: implications for prebiotic chemistry, Orig. Life Evol. Biosph. 42:31–45, 2012. Return to text.
- Imai, E., Honda, H., Hatori, K., Brack, A., and Matsuno, K., Elongation of oligopeptides in a simulated submarine hydrothermal system, Science 283:831–833, 1999. Return to text.
- Cleaves, H.J., Aubrey, A.D., and Bada, J.L., An evaluation of the critical parameters for abiotic peptide synthesis in submarine hydrothermal systems, Orig. Life Evol. Biosph. 39:109–126, 2009. Return to text.
- Martin, R.B., Free energies and equilibria of peptide bond hydrolysis and formation, Biopolymers 45:351–353, 1998. Return to text.
- Thaxton, C.B., Bradley, W.L., and Olsen, R.L., The Mystery of Life’s Origin: Reassessing current theories, Lewis and Stanley, 2nd printing, p. 142, 1992. Return to text.
- Bada, J.L., Amino Acid Cosmogeochemistry, Phil. Trans. R. Soc. Lond. B 333:349–358, 1991. Return to text.
- Brack, A., From interstellar amino acids to prebiotic catalytic peptides: a review, Chem Biodivers. 4:665–679, 2007. Return to text.
- Imai, E.-I., Honda, H., Hatori, K., Brack, A., and Matsuno, K., Elongation of oligopeptides in a simulated submarine hydrothermal system, Science 283:831–833, 1999. Return to text.
- Bada, J.L., Miller, S.L. and Zhao, M., The stability of amino acids at submarine hydrothermal vent temperatures, Origins Life Evol. Biosph. 25:111–118, 1995. Return to text.
- Ogata, Y., Imai, E.-I., Honda, H., Hatori, H.K., and Matsuno, K., Hydrothermal circulation of seawater through hot vents and contribution of interface chemistry to prebiotic synthesis, Orig. Life Evol. Biosphere 30:527–537, 2000. Return to text.
- Buckingham, D.A., Marzilli, L.G., and Sargeson, A.M., Proton exchange and mutarotation of chelated amino acids via carbanion intermediates, J. Am. Chem. Soc. 89:5133–5138, 1967. Return to text.
- Griffith, E.C. and Vaida, V., In situ observation of peptide bond formation at the water-air interface, PNAS 109(39):15697–15701, 2012. Return to text.
- Rodriguez-Garcia, M., Surman, A.J., Cooper, G.J.T., Suárez-Marina, I., Hosni, Z., Lee, M.P., and Cronin, L., Formation of oligopeptides in high yield under simple programmable conditions, Nature Communications 6(8385):1–6, 2015. Return to text.
- Rodriguez-Garcia et al., ref. 17, supplementary materials, figure 12 and table 2 Return to text.
- Rodriguez-Garcia et al., ref. 17, supplementary materials, figure 10. Return to text.
- Rodriguez-Garcia et al., ref. 17, supplementary materials, figure 9 and table 1. Return to text.
- Rodriguez-Garcia et al., ref. 17, supplementary materials, table 3. Return to text.
- Rodriguez-Garcia et al., ref. 17, supplementary materials, figure 15 and table 4. Return to text.
- Instead of varying variables at different settings as done here, where we are employed, we would have first done a mathematical DoE (Design of Experiment) in order to gain the maximum information using fewest experiments. This would have led to other reaction settings than those reported here, to cover interaction effects between variables. This would have provided optimal data to develop mathematical models for extrapolation and interpolation, and to display the effects graphically. Despite the immense amount of valuable data collected, the authors cannot tell us for sure what the best settings would be, nor what values for Glyn would result within the space of parameters they have explored. Return to text.
- If this project were of commercial interest, my employer might have financed this project for Cronin, like many other university research projects we fund. Return to text.





Readers’ comments
Comments are automatically closed 14 days after publication.