The puzzling Homo naledi: a case of variation or pathology in Homo erectus?
Introduction
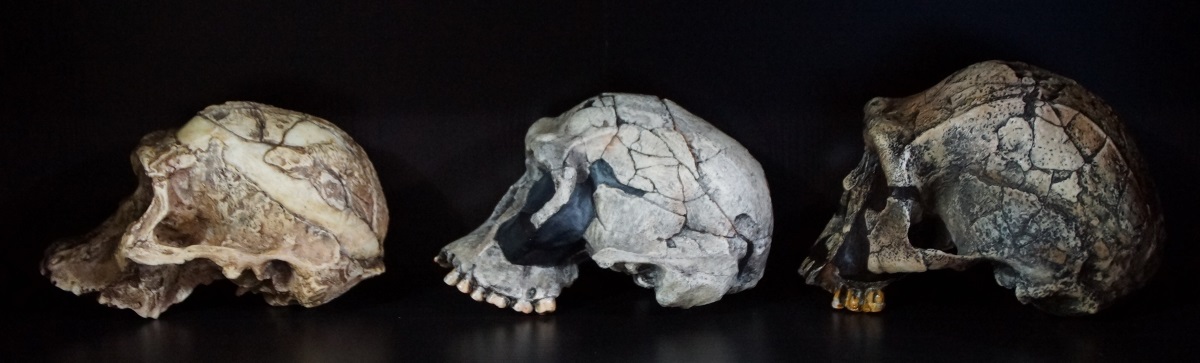
After the unearthing of Homo floresiensis (nicknamed ‘hobbits’)1 and Dmanisi Skull 5,2 one could be forgiven for thinking few such surprises in this field were left in the ground. But on Thursday 10 September 2015 paleoanthropologist Lee Berger, of the University of the Witwatersrand (Wits University), Johannesburg, South Africa, along with a team of international scientists, announced the discovery of ‘hominin’ remains they have named Homo naledi, a find that brings even more perplexity to a field still reeling from trying to make sense of the Dmanisi Homo erectus fossil collection.

Click for larger image.
It was intriguing watching live streaming video of the announcement, particularly images of the newly unveiled fossils. As someone with an interest in this area, I had been eagerly awaiting the publication of the Rising Star fossils, but I had not anticipated such a puzzling find. The announcement, at Wits University, of the ‘new species’, represented by at least 15 individuals (based on a collection of 1550 fossil elements), coincided with publication of the find in the online journal eLife.3 In a nutshell, Homo naledi is said to exhibit some anatomical features resembling those present in Australopithecus, other features resembling those in Homo, as well as several unique features. Commenting on the Homo naledi skeleton the authors state that:
“This anatomical mosaic is reflected in different regions of the skeleton. The morphology of the cranium, mandible, and dentition is mostly consistent with the genus Homo, but the brain size of H. naledi is within the range of Australopithecus. The lower limb is largely Homo-like, and the foot and ankle are particularly human in their configuration, but the pelvis appears to be flared markedly like that of Au. afarensis. The wrists, fingertips, and proportions of the fingers are shared mainly with Homo, but the proximal and intermediate manual phalanges are markedly curved, even to a greater degree than in any Australopithecus. The shoulders are configured largely like those of australopiths. The vertebrae are most similar to Pleistocene members of the genus Homo, whereas the ribcage is wide distally like Au. afarensis.”4
In addition to the Berger et al. paper describing the morphology of the Homo naledi fossils, a companion paper by Dirks et al. was also published in the eLife online journal, which described the physical context of the Dinaledi Chamber within the Rising Star cave, where the fossils were found.5 One of the many big mysteries surrounding Homo naledi is how the fossil material got into the Dinaledi Chamber, with occupation, predator accumulation and water transport hypotheses considered unlikely, but mass mortality or death trap and deliberate body disposal scenarios considered plausible—the latter explanation preferred by the authors.6 Apparently the bones recovered so far represent only “a small portion of the total fossil content”, and “no other large animal remains were found in the chamber, and … the bodies had not been damaged by scavengers or predators”.7
A note of caution
Before proceeding further, an item that deserves a brief mention relates to the initial discovery of the fossils by recreational cavers, as recounted in National Geographic:
“But it was what was on the floor that drew the two men’s attention. There were bones everywhere. The cavers first thought they must be modern. They weren’t stone heavy, like most fossils, nor were they encased in stone—they were just lying about on the surface, as if someone had tossed them in. They noticed a piece of a lower jaw, with teeth intact; it looked human.”8
That the bones “were just lying about on the surface, as if someone had tossed them in” sounds a bit suspicious, kind of makes you wonder whether this may not be what actually happened. Particularly when, as described in the same article, “It was clear from the arrangement of the bones that someone had already been there, perhaps decades before.”8
Though this makes it hard to avoid the niggling possibility of a ‘Piltdown Man’ cloud over the Homo naledi discovery, the article here assumes we are dealing with a genuine fossil find, and this author has no reason to doubt the integrity of Lee Berger and his team.
So far no stone tools have been associated with the Homo naledi fossils.9 Another mystery about the find is the ‘age’ of the bones. As discussed by Marc Ambler,10 no effort was made to date the fossils, so there is no ‘age’ for the bones, and there are indications from the ease of excavation of the fossils from soil (not lithified sediment) that the bones may be quite young. One also wonders about the extent of fossilization of the bones, that is, to what extent have organic substances in the bone been replaced with mineral substance. Of interest also is whether they will be able to extract and sequence DNA from the bones.
When studying this find one should remember that the Berger et al. analysis is based on multiple individuals, as is the ‘skeleton’ layout in the centre of the photo in Figure 1 (i.e., a composite of fossil elements representing multiple individuals) of their eLife paper, and also based on the assumption that the fossil “material represents a single species, and not a commingled assemblage”.11 Given the location of the finds this assumption is probably valid, although some paleoanthropologists, like Jeffrey Schwartz at the University of Pittsburgh, think there is too much variety in the material to represent a single species.12 (Schwartz also believes the Dmanisi fossils similarly represent more than one species.13)
What is also unclear is whether there is some sort of pathology involved, although the authors appear to have ruled this out. The reason for this is given by them in a separate Fact Sheet, where it is stated that many Homo naledi individuals all share the same unusual features, rather than varying from individual to individual, as they say would be expected if Homo naledi were a pathological modern human.14 However, this is not a very convincing argument as, for example, it may just be that the unusual features in question occur in most sufferers of the disease, while other features vary between individuals, depending on environment and genetics. Also, it may be that some of the features said to be unusual turn out not to be that unusual. The possibility of pathology in robust humans, such as Homo erectus, Homo heidelbergensis and Neandertals, needs also to be considered, not just pathology in modern humans. This possibility complicates the picture considerably. For example, a lateral flaring of the ilium of the pelvis may be considered unusual or pathological in modern humans (e.g., a feature observed in cretinism), but normal in Homo erectus.
My first impression on seeing the Homo naledi fossils was that it was some strange assemblage of Homo erectus individuals, a bit like the Dmanisi fossils, based largely on the shape of the cranium and the human-like foot, as well as some other aspects of the skeleton. In an accompanying eLife commentary article, Chris Stringer, of the Natural History Museum, London, was of the opinion that “the material looks most similar to the small-bodied examples of Homo erectus from Dmanisi in Georgia”.15 As I regard members assigned to Homo erectus as consisting of mostly humans, i.e., descendants of Adam and Eve, this analysis will investigate whether Homo naledi are a group of humans, possibly Homo erectus-like.
Before trying to make sense of the Homo naledi skeleton from a non-evolutionary point of view, one should bear in mind certain points. In order to name a new ‘hominid’ (or hominin) fossil species, the fossils have to be sufficiently different from anything else to warrant such a classification. The best way to ensure this is to emphasize attributes that give it the appearance—whether real or not—of being composed of a mixture of features present in other ‘established’ fossil species, or observed in extant humans or apes, in a combination not seen before, with perhaps some unique attributes as well.
This is what has happened in the case of Homo naledi. However, often when features are said to not be present in another species, the features are present, but at a much lower percentage in the population. Also, when comparing one fossil species to another fossil species the comparison is often not very informative, because the intra-species variation in the feature being compared is unknown due to the limited number of fossil bones available. One other point to remember is that features considered unique or ‘primitive’ may simply indicate pathology, rather than be characteristic of a new species, but there is a tendency for evolutionary paleoanthropologists to only consider the latter option. In analyzing the Homo naledi skeleton the focus here will be on features that the Berger team indicate are outside the range of humans, whether modern or robust (such as Homo erectus, Homo heidelbergensis and Neandertals). This is to see whether the features in fact really are outside human variation (including pathological explanations), in order to determine whether Homo naledi represent human individuals or australopithecine apes.
Rib cage
The rib cage of Homo naledi is described as “wide distally like Au. afarensis”.16 A little more detail is given elsewhere in the paper, as follows:
“The 11th rib is straight (uncurved), similar to Au. afarensis, and the shape of the upper rib cage appears narrow, as assessed from first and second rib fragments, suggesting that the thorax was pyramidal in shape. The 12th rib presents a robust shaft cross-section most similar to Neandertals. This combination is not found in other hominins and might reflect allometric scaling at a small trunk size.”17
The Homo erectus Nariokotome boy (KNM-WT 15000; also known as Turkana boy) is described by paleoanthropologists Alan Walker and Pat Shipman as having a barrel-shaped thorax, like us.18 Interestingly, according to paleoanthropologist Ian Tattersall of the American Museum of Natural History in New York, the Neandertal rib cage is not barrel-shaped, like in modern humans (and Homo erectus—although the range of variation in Homo erectus is not known), but an assembled entire Neandertal skeleton (consisting of fossil elements from several different sites) “boasted a conical thorax that tapered upward from the broad pelvis to a narrow top, giving it an incredibly distinctive look”.19 Before 2001, however, the Neandertal rib cage had been illustrated in textbooks to look like a “barrel-shaped human model”.20 The reconstructed rib cage of Australopithecus afarensis (represented by the famous specimen Lucy AL 288-1) is described by Walker and Shipman as being “shaped like a funnel, with the narrow part at the top and a wide lower region”.18 What the above discussion illustrates is that a wide distal (or lower region) rib cage can—apart from being interpreted to be like Australopithecus afarensis—also be interpreted as being similar to that of the Neandertals. Gene flow from Neandertals into modern humans (i.e., interbreeding) has been revealed by ancient DNA studies,21 providing further evidence of their humanity. Hence, as Neandertals are near-universally regarded as fully human, the rib cage does not preclude Homo naledi from also being human, if its rib cage is as suggested by the authors, as it would fall within human variation. However, a reconstruction “suggesting that the thorax was pyramidal in shape” does not sound convincing. Hence, at present the shape of Homo naledi’s rib cage is probably best described as indeterminate.
Shoulder

The shoulders of Homo naledi are stated as being “configured largely like those of australopiths”.22 Elsewhere in the paper the authors elaborate further by stating that the “shoulder of H. naledi is configured with the scapula situated high and lateral on the thorax, short clavicles, and little or no torsion of the humerus”.23 However, low humeral torsion is also present in the Homo erectus Nariokotome boy (KNM-WT 15000)24 and the Homo erectus Dmanisi humeri.25 Also, a relatively short clavicle has been reported for the Nariokotome boy.24 Concerning the scapula being situated high on the thorax in Homo naledi, according to evolutionists Neil Roach and Brian Richmond:
“Clavicle shape and curvature in H. erectus are also consistent with a modern-human like form. The single exception to this is Nariokotome’s increased superior clavicular curvature, which could result in a slightly more superiorly placed acromial facet and a scapula that sits higher on the thorax.”26
Hence, a scapula interpreted to be situated high is not something diagnostic of australopiths. Roach and Richmond also state that the “more complete Nariokotome scapula also shows a human-like lateral scapular spine orientation and supra- and infraspinatus fossae proportions consistent with or beyond those of modern humans”.26
Scapulae from australopiths such as Australopithecus afarensis specimen AL 288-1 and Australopithecus africanus specimen Sts 7, as well as the great apes, differ from that of modern human scapulae in having a more cranially oriented glenoid cavity, indicating habitual use of the arm in an elevated position “that would be common during climbing behavior”,27 such as suspensory arm-swinging.28 Studies of the more complete right scapula of the Nariokotome boy indicate that the glenoid cavity (or fossa) in Homo erectus was not cranially oriented; although a Dmanisi Homo erectus scapular fragment was more cranially oriented than that of the Nariokotome boy, it was still within the human range.29 The orientation of the glenoid fossa in Homo naledi does not appear to be indicated in the initial publication. However, subsequent to the publication paleoanthropologist John Hawks, from the University of Wisconsin–Madison, a senior researcher in the Berger group, stated in a weblog that: “The H. naledi scapula has a superiorly oriented glenoid, very different from the Dmanisi scapula specimen or the Nariokotome H. erectus skeleton.”30 If the relevant parts of the Homo naledi scapula are present, I presume that in a forthcoming paper the Berger team will publish measurements, such as the angle between the glenoid cavity and the axillary (lateral) border of the scapula (the gleno-axillary angle), and/or the angle between the ventral bar and the glenoid cavity, which will allow a more objective assessment of Homo naledi’s glenoid cavity orientation.
Hand
In the initial paper by Berger et al. the different parts of the hand of Homo naledi are summed up as follows: “The wrists, fingertips, and proportions of the fingers are shared mainly with Homo, but the proximal and intermediate manual phalanges are markedly curved, even to a greater degree than in any Australopithecus.”31 Homo is a very broad term, but the authors are a little more specific about the hand of Homo naledi elsewhere in the paper, when they state: “The hand shares many derived features of modern humans and Neandertals in the thumb, wrist, and palm, but has relatively long and markedly curved fingers.”32
Also, in the discussion it is commented that: “The H. naledi hand shares aspects of Homo morphology in the wrist, thumb and palm, pointing to enhanced object manipulation ability relative to australopiths, including Au. sediba.”33 A picture of the hand is shown in the October issue of National Geographic, with the caption stating that: “H. naledi’s hand displays curved fingers, a clue that the species had retained an ability to climb in trees and on rocks. The thumb, wrist, and palm bones all look remarkably modern.”34
Hence, most of the hand is human-like, except for the curved fingers, perhaps suggesting Homo naledi was suited for climbing. This is because degree of phalangeal curvature appears to be associated with functionality during ontogeny. In a study on the biomechanics of phalangeal curvature paleoanthropologist Brian Richmond concluded that:
“the strain differences between curved and straight phalanges illustrated here support the common assertion that phalangeal shaft curvature is related to the strains associated with arboreal and especially suspensory activity … and may underlie changes in curvature during ontogeny in response to changes in mechanical environments of arboreal and terrestrial supports”.35
It is interesting that when analysis of Australopithecus sediba’s hand was published it was reported, concerning proximal phalangeal curvature, that:
“Though there is substantial overlap in the degree of curvature across numerous taxa, Au. sediba curvature is less than that of Ar. kadabba, Au. afarensis, the OH7 phalanges … and a Swartkrans specimen tentatively attributed to H. cf. erectus.”36
Even as it is admitted by the Berger group that the manual (hand) morphology of Homo erectus “is largely unknown”,37 it is interesting that one specimen (a proximal phalanx SKX 27431), that they earlier “tentatively attributed to H. cf. erectus”, exhibits marked dorsal phalangeal curvature (well within the Australopithecus afarensis and chimpanzee range).36 Whether SKX 27431 actually belonged to a Homo erectus specimen is not clear, but if it did, then curved fingers would not be a feature separating Homo naledi from Homo erectus. Adding to the confusion, previous analysis, led by paleoanthropologist Randall Susman, of the University at Stony Brook, found “essentially humanlike curvature” in a proximal fossil phalanx (SKX 5018)38,39 from Swartkrans, South Africa, tentatively attributed to Australopithecus robustus (a robust australopithecine).36 Also, other studies have shown there is some overlap between the upper and lower end of the ranges of curvature in the fingers (e.g., in the proximal manual phalanges) of modern humans (Homo sapiens) and chimpanzees (Pan troglodytes) respectively.40,41 During the writing of this paper a publication on the hand of Homo naledi was produced by Kivell et al., which although providing a lot more detail, essentially told the same story as the initial paper:
“Our comparative analyses reveal that the wrist and palm are generally most similar to those of Neandertals and modern humans, while the fingers are more curved than some australopiths. This distinctive mosaic of morphology has yet to be observed in any other hominin taxon and suggests the use of the hand for arboreal locomotion in combination with forceful precision manipulation typically used during tool-related behaviours.”42

In their graph reflecting phalangeal curvature there appears to be no overlap between the ranges of modern humans and chimpanzees, nor between modern humans and Homo naledi.42 Hence, it is very unlikely that the degree of phalangeal curvature exhibited by Homo naledi can be explained by normal human variation. However, there appears to be something very strange about the curvature of Homo naledi’s fingers, and that is the high degree of curvature of not just the proximal phalanges (PPs), but also the intermediate phalanges (IPs). The authors state that:
“The mean curvature of the IPs (n=14) is higher than that of any other hominin and not statistically distinct from Asian apes (Fig. 7). Although there is variation across fossil hominins, a combination of both highly curved PPs and IPs is unusual; extant apes and most fossil hominins, such as A. afarensis and OH7, generally have more strongly curved PPs and comparatively straight IPs.”
At face value the fingers of Homo naledi appear better suited to climbing than chimpanzees, as the PPs are about the same curvature, but Homo naledi’s IPs are considerably more curved (the median value even higher than orangutans). Yet, other aspects of Homo naledi’s hand “look remarkably modern”, indicating that the hands probably belonged to a human. This does not appear to make sense in any scenario, whether creation or evolution. Something is not quite right. If indeed the hand is from a human, then a likely explanation for the curved fingers is some sort of bone pathology.
It is interesting that “Mozart’s fingers were extremely curved, and that this was possibly due to bone deformities suggestive of old [healed] rickets”, as apparently the lack of light and dietary conditions of his infancy left little doubt that “Mozart must have suffered from rickets or at least from vitamin D deficiency”.43 Could it be that Homo naledi individuals were, at least in their infancy, prone to suffer from vitamin D deficiency and consequent rickets, from lack of sunlight and low vitamin D content in their diet? This would result in bone abnormality or deformities, as well as growth retardation.44 Perhaps, when the children were young, they spent considerable time in caves for safety reasons, resulting in little exposure to sunlight? As they got older they were allowed out more, and hence exposed to sunlight more, which may have healed them from the rickets, but curved fingers remained as scars from the childhood condition. They may also have been deeply pigmented individuals, for all we know, resulting in inadequate penetration of whatever light they were exposed to. These are of course speculative suggestions, but so are a lot of things about Homo naledi.
However, there is another, even more plausible explanation. It is very interesting that in regards to Homo floresiensis “the proximal phalanges are curved to a similar degree as in Au. afarensis”.45 The proximal phalanx referred to belongs to the LB6 Homo floresiensis individual. The authors of the publication that performed the study commented that “LB6/8 falls at the extreme upper end of the human range and overlaps with gorillas. It is similar in this respect to A.L. 333w-4, an Australopithecus afarensis specimen”.46 Note that the proximal manual phalanges (i.e., proximal finger bones) of the Homo floresiensis LB1 individual are not complete enough to make conclusive judgment on curvature, but it is said the “shafts appear to be relatively straight”.46 No information appears to be given on the curvature of the intermediate manual phalanges of the LB1 and LB6 Homo floresiensis individuals.46 The species designation of Homo floresiensis has been controversial, as it has been argued by some evolutionists that it instead consists of individuals, such as LB1 and LB6, that “are, most likely, endemic cretins from a population of unaffected Homo sapiens”.47 Hence, did the Homo naledi individuals suffer from cretinism, in a similar way that individuals from the Homo floresiensis species possibly did, with the curved fingers related to cretinism or associated conditions?
Pelvis
In the National Geographic article about Homo naledi, by Jamie Shreeve, the hip bones of Homo naledi in the composite skeleton are said to “flare outward—a primitive trait—and are shorter front to back than those of modern humans”.48 The Berger group elaborate a little bit more on the pelvis of Homo naledi, as follows:
“The Dinaledi iliac blade is flared and shortened anteroposteriorly, resembling Au. afarensis or Au. africanus. The ischium is short with a narrow tuberoacetabular sulcus, and the ischiopubic and iliopubic rami are thick, resembling Au. sediba and H. erectus. This combination of iliac and ischiopubic features has not been found in other fossil hominins”.49
Focusing on how the Homo naledi pelvis differs from that of Homo erectus, John Hawks states in his weblog that “the pelvis of H. naledi exhibits a short, flared ilium unlike those known for H. erectus, including the Gona pelvic specimen”.30 There are pelvic bones attributed to Homo erectus that are described as having “broad, laterally flaring ilia”, including the Gona specimen (BSN49/P27), OH 28 and KNM-ER 3228.50 According to Laura Gruss the “pelvis of H. erectus, while broad compared with modern humans, was narrower relative to body height than in the australopithecines”.51 The australopithecine ilium has been described as “excessively broad”, such that the “breadth of the human iliac blade is actually intermediate between those of the chimp and of Australopithecus”.52
Since the Berger et al. paper describes the Homo naledi pelvis as appearing to be “flared markedly like that of Au. afarensis”,53 with the main example of the latter (the Lucy pelvis) described elsewhere as exhibiting a “pronounced lateral flare” of the iliac blade,54 one presumes that Homo naledi had laterally flaring ilia. Hence, what appears to differentiate the Homo naledi and Homo erectus ilia is the description of the former as being a short, flared ilium, rather than being a broad, flared ilium, the description of the latter. However, given the few Homo erectus pelvises with which to compare, one wonders whether, rather than being evidence in support of a new species, any such alleged difference in the Homo naledi pelvis may simply reflect how little information there is on the postcranial diversity within people labelled Homo erectus. It could perhaps even be related to the pelvic elements of Homo naledi coming from small-sized individuals, or even pathology. One awaits future publication on the pelvis of Homo naledi, which will hopefully provide more information.
According to the Berger group the pelvis of Homo naledi “appears to be flared markedly like that of Au. afarensis”.53 Similar to the description of the Homo naledi pelvis, it has been stated in regards to the pelvis of the Homo floresiensis type specimen (LB1) that its “marked degree of lateral iliac flaring recalls that seen in australopithecines such as ‘Lucy’ (AL 288-1)”.55 As opposed to being laterally flared, in modern humans the iliac blades curve or wrap around the sides of the body more. As already mentioned, even some evolutionists believe individuals from Homo floresiensis were actually pathological humans, with cretinism a plausible explanation.56 Interestingly, one of the features noted in cretinism is lateral flaring of the ilium of the pelvis.57
Foot
Assessing Homo naledi the Berger group state that “the foot and ankle are particularly human in their configuration”.58 Essentially the only traits of its foot regarded as “primitive” are evidence “suggestive of a lower arched foot”59 and “slightly more curved toe bones”.60 Even so, paleontologist Will Harcourt-Smith, who was lead author on a subsequent publication on the Homo naledi foot61 (although providing a lot more detail, this paper told essentially the same story as the initial paper), says that it “is essentially the foot of a modern human, but subtly different”.60 Paleoanthropologist Dan Lieberman is quoted as saying: “The foot is indeed strikingly modern … and suggests it walked and possibly ran much like modern humans.”62 The foot of Homo naledi was not compared to that of Homo erectus because foot morphology of the latter was stated as “too poorly known to allow for comparison”.63
According to evolutionary experts: “All primates possess a transverse arch, but only humans have a longitudinal arch making non-human primates anatomically and functionally flat-footed.”64 The longitudinal arch is a structure involved in storing elastic energy and it “maintains the structural rigor of the foot during the push-off stage of bipedal locomotion”.64 As for the lower-arched foot, the Berger group state in their separate Fact Sheet that Homo naledi “likely had minimally developed longitudinal foot arches (i.e., flatter feet), which is uncommon (but not unknown) in living people”.65 According to foot and ankle specialists, flatfoot is a frequently encountered pathology in both pediatric66 and adult67 human populations. According to these specialists “adult flatfoot is defined as a foot condition that persists or develops after skeletal maturity and is characterized by partial or complete loss (collapse) of the medial longitudinal arch”.67 It is said to encompass “a wide variety of pathologic etiologies” and to often be “a complex disorder with a diversity of symptoms and various degrees of deformity”.67 The point is that if less developed longitudinal foot arches are not regarded as ‘primitive’ in the modern human foot, then neither should it be in the foot of Homo naledi, described as “essentially the foot of a modern human”, but having “features that may signal a relatively low medial longitudinal arch, at least in Foot 1”.68 It is also interesting to note that, according to Jungers et al., in Homo floresiensis the big toe (hallux) was fully adducted (in line with the rest of the foot), but a medial longitudinal arch was suspected to be absent.69 Hence, Homo floresiensis probably had flatter feet than Homo naledi.
The Berger group Fact Sheet mentions human-like features of Homo naledi, for example, that their “big toes were in-line with the rest of the foot, unlike the grasping, opposable big toe in chimps”, but also mention that their “toes were also slightly curved—not as much as a chimp’s toes—but more than in humans”.70 Some overlap has been shown between the upper and lower end of the ranges of curvature in the toes (e.g., in the proximal pedal phalanges) of modern humans and chimpanzees respectively.71 In the subsequent publication on the Homo naledi foot the range of curvature in the pedal proximal phalanges of Homo naledi appear to overlap considerably with Homo sapiens, although not completely so.72 This is very different from the manual proximal phalanges, where there was essentially no overlap. It is, however, a little bit odd that the toes of Homo naledi are slightly more curved than those of modern humans. This is because it doesn’t appear to reflect any functionality. To be used effectively for climbing in trees the feet of Homo naledi would need to have a grasping, opposable big toe as chimpanzees do, but Homo naledi’s big toe was in line with the rest of the foot, like in humans.
As essentially everything else about the foot was modern human-like, one possible explanation of the slight curvature in the toes of Homo naledi may be, similar to the explanation of finger curvature, that they belonged to human individuals who were, at least in their infancy, prone to suffer from Vitamin D deficiency leading to rickets, from lack of sunlight and low dietary vitamin D (as noted earlier for the hand). However, other explanations are also plausible. For example, as with the finger bones, the toe bones of Homo floresiensis are said to be slightly curved (i.e., of the proximal pedal phalanges).69 As already mentioned, Homo floresiensis is associated with cretinism.
Other postcranial skeletal parts
Aspects considered ‘primitive’ in the rib cage, shoulder, hand, pelvis and foot have been discussed above. Most other parts of the postcranial skeleton are generally human-like, whether it be like modern humans and/or ‘robust humans’, the latter being those humans that are broadly categorized as Neandertals, Homo heidelbergensis or Homo erectus. Concerning the vertebrae:
“The preserved adult T10 and T11 vertebrae are proportioned similarly to Pleistocene Homo, with transverse process morphology most similar to Neandertals. The neural canals of these vertebrae are large in comparison to the diminutive overall size of the vertebrae, proportionally recalling Dmanisi H. erectus, Neandertals, and modern humans.”73
Hence, the vertebrae are consistent with Homo naledi being human. Based on a tibia (U.W. 101-484), the stature of one Homo naledi individual was estimated to be just under 1.5 m (4ft 9in), whereas body mass was estimated, from eight femur specimens, to vary from about 40 kg to 56 kg (90 lb to 120 lb); with estimates of both stature and body mass “similar to small-bodied modern human populations”.74 For whatever reason no information on limb proportions in Homo naledi, such as humerofemoral index (humerus length/femur length), brachial index (radius length /humerus length), or crural index (tibia length/femur length) appear to be given in the paper, but it is stated that locomotor “traits shared with Homo include the absolutely long lower limb”,75 which is consistent with Homo naledi being human-like. Homo naledi is said to possess a valgus knee76 (angling inward of the femur making the knees closer together), a characteristic of humans, which is associated with efficient bipedalism.
Under seeming pressure to differentiate Homo naledi from Homo erectus, much fuss has been made about its femoral neck being relatively long and anteroposteriorly compressed,77 a feature allegedly making it look different from African and Dmanisi femora attributed to Homo erectus.30 It is generally considered an “archaic morphology”78 (i.e., femoral necks that are narrow anteroposteriorly relative to superoinferiorly), as it is considered typical of the australopithecines, but not in modern humans or femora attributed to Homo erectus.79 While as a group the femoral neck of australopithecines is statistically anteroposteriorly compressed compared to modern humans, data from Christopher Ruff and Ryan Higgins indicates that individually quite a few of the femora from the modern human sample are similarly anteroposteriorly compressed.80 Hence, if this feature is not unique to the australopithecines, but also present in modern humans, albeit less frequently, it is not an “archaic morphology” that supports assignment of Homo naledi to a new species of ‘ape-man’. Ruff and Higgins had two femora (KNM-ER 1472 and KNM-ER 1481) attributed to Homo erectus as part of their analysis.81 These were not anteroposteriorly compressed, and even if the Dmanisi femur is not either, then this only leaves a sample size of three—hardly enough to establish the range of intra-species variation.
Skull
The skull of Homo naledi is given considerable attention in the Berger group paper, including comparisons with other ‘fossil’ species and Homo sapiens. The holotype specimen (presumed male) is called Dinaledi Hominin 1 (DH1), comprising a partial calvaria (the cranium minus the face), partial maxilla and a nearly complete mandible, and assigned paratypes comprising skull bones are DH2 (partial calavaria), DH3 (partial calvaria and partial mandible of a relatively old individual, presumed female), DH4 (partial calvaria), DH5 (partial calvaria) and U.W. 101-377 (mandibular fragment).82 In a nutshell, the authors summarize the Homo naledi skull by saying the “morphology of the cranium, mandible, and dentition is mostly consistent with the genus Homo, but the brain size of H. naledi is within the range of Australopithecus”.83 Hence, there are two basic issues, the morphology of the cranium and brain size, and the former will be considered first.

The skull being “mostly consistent with the genus Homo” is not really that informative, given the diversity within this group. In their paper the authors compare the Homo naledi skull with those from various australopithecines, as well as Homo habilis, Homo rudolfensis, Homo erectus, Homo floresiensis, Homo sapiens, and a group consisting of Homo antecessor, Homo heidelbergensis, Homo rhodesiensis, archaic Homo sapiens and Neandertals. Despite this smorgasbord of species (real or otherwise) to choose from, surprisingly Homo naledi could not be incorporated into any of them. In the South African Journal of Science, Patrick Randolph-Quinney describes the Homo naledi skull as follows:
“ The teeth of H. naledi are generally small, with simple cusp morphology—traits shared with Homo habilis. The skull morphology of H. naledi is unique, but shares similarities to other early Homo species including H. erectus, H. habilis and H. rudolfensis, and differs markedly from taxa such as Paranthropus and Australopithecus ghari through lack of cranial crests, and A. afarensis, A. africanus and A. sediba through (amongst others) the expression of sagittal keeling and an angular occipital torus, and in the brow region with a pronounced supraorbital torus with post-toral sulcus (a depression between the brow ridge and the rising frontal bone). The mandible is gracile, with a vertically oriented symphyseal region, overall more akin to early Homo than Paranthropusor Australopithecus.”84
When the Homo erectus Dmanisi Skull 5 was revealed in 2013 (comprising the D4500 cranium and D2600 mandible),2 one of the big surprises was the implication of this find on the variability of Homo erectus, at least of the skull, with the morphological variation considered wide indeed.85 Given that there is enormous variation in the skulls of specimens labelled Homo erectus, is the skull of Homo naledi really that different? After the publication of the Homo naledi finds there has been debate between some evolutionary paleoanthropologists about whether the fossil specimens, rather than being assigned to the new species Homo naledi, could have instead been subsumed within the existent species Homo erectus. When asked whether Homo naledi could be considered a new species without dating, paleoanthropologist Tim White responded as follows:
“Dating is irrelevant; these are a small, primitive H. erectus, whatever the date turns out to be. This is because they are not biologically different, in any significant way, from already known H. erectus found in places like Swartkrans (800m away), eastern Africa, or the Georgian republic. Of the 80+ traits listed in the e-LIFE supplemental material, only a small fraction of them are even claimed to differentiate these fossils from earlier described H. erectus, and that fraction of characters is known to vary among members of the same species (even population) of both H. erectus and H. sapiens. In other words, the newly described ‘species’ is an example of artificial species inflation in palaeoanthropology.”86
The 80-plus traits mentioned by White were cranial and mandibular characters. John Hawks, one of the authors of the eLife paper and senior member of the Berger team, responded in a weblog article to Tim White’s criticism by saying:
“White doesn’t specify how many features would be sufficient in his view to define a species. It should be obvious that if we list 80 cranial and dental traits that are informative among all hominins, that most species will differ in only a relatively small fraction of those. Nevertheless our open access paper lists many clear differences between H. naledi and H. erectus, including aspects of premolar crown and root morphology, the crown morphology of the molars (simplified in H. naledi, invariably crenulated and complex in H. erectus), vault shape (H. naledi does not have the elongated, low cranium of H. erectus) and mandibular shape—all listed on page 10 of our open access paper. Even setting aside the postcranial skeleton and the very small endocranial volume, these show H. naledi to be distinct from H. erectus.”30
Hence, according to Hawks et al. (and on page 10 in their paper) “H. naledi does not have the elongated, low cranium of H. erectus”. In Chris Stringer’s accompanying eLife article a cast of a Homo naledi cranium is compared to other casts, including Homo erectus, with typical features of each species labelled on the crania.87 Homo naledi is labelled as having a “relatively high and thin skull” and small teeth, whereas Homo erectus is labelled as having a “relatively low and thick skull” and large teeth, with both having a flexed (angulated) occipital and transverse torus.87
The Berger paper states that “compared to samples of H. habilis, H. rudolfensis, and H. erectus, the teeth of H. naledi are comparatively quite small, similar in dimensions to much later samples of Homo”.88 Having small teeth is a feature of modern humans, as is having a high and thin skull. Also, the cranial vault of Homo naledi is described as having only slight post-orbital constriction, the mandibular dental arcade as parabolic in shape, and the mandibular corpus (body) as being relatively gracile.89 These features of the skull do not align it with the australopithecines, but rather indicate that it is a human skull, albeit not an anatomically modern human skull. In National Geographic, the general shape of the composite male Homo naledi skull is said to be ‘advanced’, as well as labelled a ‘Humanesque skull’.90 Interestingly, when commenting on the skulls of Homo naledi paleoanthropologist Jeffrey Schwartz states that:
“Viewed from the side, two partial skulls are long and low, with a long gently sloping forehead that flows smoothly into the brow—nothing like us, or most specimens regarded as Homo. A third partial skull is very short and rounded, with a high-rising forehead that is distinguished from a distinct, well-defined brow by a shallow gutter—not like the other skulls, and not like us or most specimens regarded as Homo.”91
However, according to Hawks: “Schwartz is also totally wrong about the crania: All the frontal bones in the collection have a similar morphology with a thin supraorbital torus and slight but distinct supratoral sulcus.”30 Having had close access to the Homo naledi fossils, perhaps Hawks is correct about the crania all being similar in morphology, but there is indisputable evidence that the morphology of skulls classified by evolutionists as Homo erectus vary considerably, a point illustrated by Schwartz et al. in an earlier publication.92 Hence, when considering this diversity, and whether Homo naledi belongs with this motley assortment of crania classified as Homo erectus, one could hardly exclude it from membership based on appearance. However, regardless of whether it is classified as Homo erectus or not, the form of the skull appears to be within human variation (here human variation encompasses the combined range of both modern and robust humans). This brings us to its cranial capacity, which is perhaps the most astonishing aspect about Homo naledi, and probably the main justification for putting it in a separate species.
Cranial capacity
Homo naledi is said to be “characterized by body mass and stature similar to small-bodied human populations but a small endocranial volume similar to australopiths”.93 Note that brain size correlates with the cranial capacity, i.e. the volume of the cranial cavity (endocranial volume), measured in cubic centimeters (cc), although in actuality it is slightly less, as not all of the cavity is occupied by the brain. In regards to cranial capacity the authors state:
“We combined information from the most complete cranial vault specimens to arrive at an estimate of endocranial volume for both larger (presumably male) and smaller (presumably female) individuals…The resulting estimates of approximately 560cc and 465cc, respectively, overlap entirely with the range of endocranial volumes known for australopiths. Within the genus Homo, only the smallest specimens of H. habilis, one single H. erectus specimen, and H. floresiensis overlap with these values.”94
Details of the virtual reconstruction of the smaller (DH3 and DH4) and larger (DH1 and DH2) composite crania, and the virtual reconstruction of cranial capacity, is given in the material and methods section of the Berger group paper.95 Apart from the merger of crania from different specimens, a big problem with the cranial capacity values of Homo naledi appears to be the amount of guesswork involved, evident by reference to the number of holes (large and small) filled by various software functions. Large parts of both composite skulls are missing including, for example, most of the cranial base in the smaller DH3/DH4 composite cranium (465 cc), and most of the frontal region in the DH1/DH2 composite cranium (560 cc). As a reality check, for the smaller composite crania (DH3/DH4), missing most of its cranial base, the authors also merged it with a scaled virtual model of the cranial base from fossil specimen Sts 19 (assigned to Australopithecus africanus). There was high correlation between the two separate cranial capacity estimates for the DH3/DH4 crania, but instead of validating the Homo naledi cranial capacity, perhaps using an australopithecine cranial base instead just confirms that the filled in portions were good at assuming an australopithecine cranial base to begin with. But does it really matter?
The example of the KNM-ER 1470 cranium (allocated to either Homo habilis or Homo rudolfensis) illustrates how it can matter. Originally reconstructed based on the preconception that it had a more human-like vertically oriented face, one that “emphasized the large brain”,96 its cranial capacity was originally estimated at 810cc.97 Later, after some basal portions reconstruction, a new estimate of 752 cc was made.98,99 However, a later more realistically based craniofacial reconstruction, yielding a relatively prognathic skull, gave KNM-ER 1470 a cranial capacity of 700 cc.100 Before this latest estimate, by researchers led by facial bone development expert and paleoanthropologist Timothy Bromage, news of them having obtained cranial capacity values as low as 526 cc were reported,101 with 575 cc even mentioned in the journal Science.102 However, the lowest values later appear to have been discarded. Ignoring the low 526 cc value, the difference between the original value and the one reported in Science was 235 cc. This is a considerable difference in cranial capacity estimates for the same cranium. In the case of Homo naledi, a smaller australopithecine-like cranial capacity makes the argument that it should be lumped in with Homo erectus harder to make, and so supports a new species hypothesis. There is no suggestion that the cranial capacity of Homo naledi was intentionally minimized, but there is the possibility that preconceived notions about cranial capacity, or preferred cranial capacity, may have influenced reconstruction of the composite skulls.
Before Homo naledi, the smallest estimate of cranial capacity of a Homo erectus skull from Africa, at 691 cc, was KNM-ER 42700, believed to be of “a young adult or a late subadult”.103 Outside Africa, smaller cranial capacities have been estimated from Dmanisi, Georgia. The cranial capacity of 546 cc for Dmanisi Skull 5 (D4500/D2600) is the smallest of the Dmanisi sample, with cranial capacities of the other four skulls reported to be between 601 cc to 730 cc.104 Specifically, David Lordkipanidze et al. list 730 cc for skull D2280, < 650 cc for skull D2282/D211, 601 cc for skull D2700/D2735, and 641 cc for skull D3444/D3900 (note the first number refers to the cranium, and the second number to the linked mandible).105 Initially the cranial capacity of cranium D2280 was estimated at 775 cc,106 whereas now it is given as 730 cc. The cranial capacity of the Homo habilis hypodigm is given to be from 509 cc to 687 cc.107 For comparison the cranial capacity range estimates for the australopithecines (including the robust australopithecines) is from about 387 cc to 560 cc.108 Some evolutionists, such as Bernard Wood and Mark Collard, have suggested that the species Homo rudolfensis (exemplified by cranium KNM-ER 1470) and Homo habilis (exemplified by cranium KNM-ER 1813) be transferred from the genus Homo to Australopithecus, to become known as Australopithecus rudolfensis and Australopithecus habilis respectively.109 Whether they warrant separate species designation is debatable, but that most of the specimens in these species belong with the australopithecines is also the opinion of this author. The mean cranial capacity for modern humans is about 1345 cc, but the range of modern humans able to function normally is difficult to specify, although approximately 700 cc to 2200 cc is given by expert Stephen Molnar, who comments that “there are many persons with 700 to 800 cubic centimeters”.110
Other Homo erectus crania, such as the ‘late’ Javanese Ngandong ‘Solo Man’ series crania (1013 cc to 1251 cc) and ‘early’ Javanese Trinil and Sangiran series crania (813 cc to 1059 cc), from Indonesia, both exceed the Dmanisi range, although are still below the mean modern human cranial capacity.111 Of other interest is the LB1 Homo floresiensis cranium, most recently estimated to be 426 cc.112 Interestingly, there have also been inconsistencies in the published cranial capacity values for LB1 (380 cc to 430 cc).112 If, as considered here, the australopithecines were extinct ‘apes’, and Homo erectus and other ‘robust’ humans were fully human, that is, descendants of Adam and Eve, where does Homo naledi fit in? That is the focal point of discussion leading to the conclusion, next.
Discussion and conclusion
Whatever one believes Homo naledi to be, it is undoubtedly an intriguing fossil find. Firstly, why cannot Homo naledi have been an ape-man, in transition from australopithecine to Homo erectus? One reason is the impossible probability of an australopithecine skeleton evolving into a Homo erectus skeleton. For example, to generate the anatomical changes necessary to make an australopithecine walk and run like a human would require many precisely coordinated genetic mutations. Ann Gauger, an expert in Developmental Biology, looked at this issue, and in looking at the odds of it happening concluded: “Given these numbers, it is extremely improbable, if not absolutely impossible, for us to have evolved from hominin ancestors by a gradual, unguided process.”113
Evolution is a construct of the human mind, and in reality it does not explain how information was delivered into the genome. Also, as demonstrated by geneticist John Sanford, the problem is even worse, as the genome has been deteriorating (due to accumulation of genetic mutations) ever since its origin, with the proposed evolutionary mechanism of natural selection sorting random mutations powerless to stop it.114 It has been conservatively estimated that in human reproductive cells the accumulation of mutations is at least 100 point mutations per person per generation.115 Hence, at this rate, in addition to evolution not being able to explain the arrival of information, it cannot explain the preservation of information over timespans of millions of years.
Can Homo naledi be human? As discussed earlier, most of the features that are said to be ‘primitive’ in Homo naledi are still within human variation, whether it be modern humans or robust humans (e.g., Homo erectus, Homo heidelbergensis and Neandertals). If Homo naledi is human, the features most difficult to explain are the small cranial capacity and the curved fingers of the hand. With regards to the curved finger and toe bones, a comparison of Homo naledi with Homo erectus is difficult because there is not enough fossil material of the latter to allow for comparisons of the phalanges.116 The finger curvature of Homo naledi certainly appears outside the range of non-pathological modern humans, but how do we know that Homo naledi individuals did not suffer from some pathology? As discussed earlier, if the human-like hand of Homo naledi is from a human, then a possible explanation for the curved fingers is some sort of bone pathology, possibly vitamin D deficiency and/or old rickets, Another explanation, regarded as the more plausible by this author, is that it may be associated with cretinism, which is also a non-genetic condition causing bone pathology (see below discussion on Homo floresiensis).
In paleoanthropologist Tim White’s eye, “Berger’s findings are probably South African representatives of Homo erectus. The Homo naledi cranium is similar in conformation and size to the earliest and most primitive Homo erectus representatives”.117 Hence, as discussed earlier, and also in the opinion of other evolutionary experts, the cranium of Homo naledi is within the Homo erectus range of variability. An unusual aspect of the cranium, however, is its diminutive cranial capacity, which is small even for Homo erectus. Although, as already discussed, there are doubts about the accuracy of the estimated cranial capacity values of the Homo naledi composite skulls (465 cc and 560 cc for DH3/DH4 and DH1/DH2 crania respectively), there is no doubt they are very small. As already indicated, the cranial capacity of Homo naledi is not within the range of what could be considered normal for a modern human. One of the smallest brain sizes documented of a modern human with normal intelligence was from Daniel Lyon, a man of small stature (height of 1.55 m [about 5 ft]), with a brain that weighed 680 g, yielding a brain volume of 624 cc, as calculated by John Skoyles.118 Using a formula linking brain volume to cranial capacity, the cranial capacity of Lyon’s cranium can be estimated to be about 660 cc.119 According to Skoyles the only unusual feature about Lyon’s brain “was that the cerebellum was near normal size”, with his cerebral hemispheres of 371 cc being “128 cc less than the 499 cc (80%) which would be expected of a normally proportioned brain of 624 cc”.118 It is interesting that if the remainder 20% was proportioned according to Lyons’ small cerebral hemispheres then his brain volume would have been about 464 cc, yielding a cranial capacity of approximately 489 cc. How proportioning the brain this way would impact brain function is unknown, but in theory, at least, it may indicate that very small individuals, like those of Homo naledi (estimated to be just under 1.5 m (4 ft 9 in) tall), could possibly have functioned relatively normally with their small brains.
If Homo naledi are just small-brained Homo erectus specimens, are they part of the normal variation of these robust humans? Given the number of Homo erectus specimens with small cranial capacities this phenomenon surely reflects something intrinsic about these humans. It is hard to escape the conclusion that the range of what could be considered normal brain size would have been lower in Homo erectus compared to modern humans. However, it should be remembered that other robust humans, such as the Neandertals, had generally quite large brains compared to the average modern human brain. One explanation why humans, such as Homo erectus and Neandertals, were more ‘robust’ or different in morphology to modern humans is that it could reflect changes in development of pre-Flood and early post-Flood individuals, linked to longevity, possibly involving thyroid hormone secretion patterns as the primary mechanism causing the change.120 Whether Homo naledi individuals should be considered normal for Homo erectus, except for, perhaps, having suffered in infancy from vitamin D deficiency and/or rickets, is difficult to answer definitively. The only comparable skulls in terms of cranial capacity are 546 cc for the Skull 5 (D4500/D2600) Dmanisi Homo erectus cranium and 426 cc for the LB1 Homo floresiensis cranium, both considered by this author to be robust humans with cretinism.85
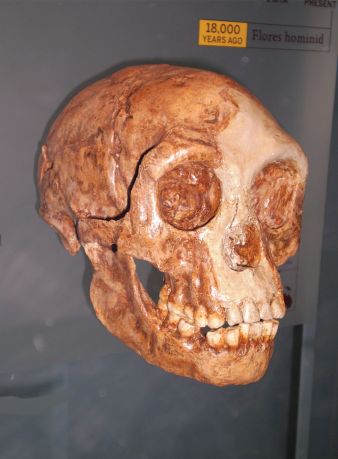
As already mentioned, in regards to Homo floresiensis, some evolutionists have argued that it shows similarities to hypothyroid endemic cretins “from a population of unaffected Homo sapiens”.47 Cretinism brought about by environmental iodine deficiency (cretins being the offspring of mothers with severe iodine deficiency) is not a genetic disorder,121 and can occur anywhere there is iodine deficiency in the food chain. As such it can affect entire populations in an environment where iodine deficiency is endemic, and people in different parts of the world, although “morphological traits vary substantially”.122 Cretinism (congenital hypothyroidism) “can reduce brain size by approximately 50%”.123 Hence, while cretins from modern human populations of large brain size may not give rise to cretins with small enough brains to explain Homo naledi or Homo floresiensis, parent populations with smaller brains, such as Homo erectus humans, could do so. Most likely so would also the small-brained Homo sapiens population from Palau, Micronesia,124 but if individuals assigned to Homo naledi and Homo floresiensis are cretins, then it makes more sense that they come from robust human populations, such as Homo erectus, because of their similarity in skeletal features to the latter. The similarities of Homo naledi to Homo erectus have been discussed throughout this paper, and in regards to Homo floresiensis, in the original publication announcing the find it was suggested that it was the result of “endemic dwarfing, of an ancestral H. erectus population”125
Apart from small brain size and stature, some of the skeletal features found in Homo naledi that have been discussed earlier, which are also noted in Homo floresiensis, are: lateral flaring of the ilium of the pelvis,69 relatively short clavicle,126 low humeral torsion,126 reduced medial longitudinal arch (i.e., flatter feet; actually arch suspected to be absent in Homo floresiensis),69 curved finger bones,127 and slightly curved toe bones.128 Some of these features have also been documented in modern humans with cretinism, including lateral flaring of the ilium of the pelvis,129 relatively short clavicle,47 and low humeral torsion,123 whereas the presence of other features is unclear—due to a lack of information.
However, it should also be pointed out that one would not expect all cretins to have the same features, particularly ones living as far apart as in Africa and Indonesia (e.g., if Homo floresiensis and Homo naledi are cretins). This is because “cretins are enormously more variable than unaffected humans in many features (as would be expected in a pathology with different degrees of affect [sic], and conflation with associated conditions)”.130 According to Charles Oxnard “all cretins are not identical. The effects of the deficiency vary to greater or lesser degree. Their genetic heritages can also be expected to influence the picture”.131 It would not be that surprising if some alleged hominids were instead robust humans that had suffered from cretinism, given that many features of cretinism mimic so-called ‘primitive’ features of evolution. Evolutionist Oxnard makes the following revealing statement:
“It is remarkable that so many features similar to those normally present in great apes, in Australopithecus and Paranthropus, and in early Homo (e.g., H. erectus and even to some degree, H. neanderthalensis) but not in modern H. sapiens are generated in humans by growth deficits due to the absence of thyroid hormone. In other words, many of the pathological features of cretinism mimic the primitive characters of evolution making it easy to mistake pathological features for primitive characters. The differences can be disentangled by understanding the underlying biology of characters.”132
If a modern human with cretinism can have many pathological features that mimic the so-called ‘primitive’ features of evolution, it is highly likely that robust humans, such as Homo erectus, with cretinism will have as many, if not even more such features. If indeed some (or most) of the Homo naledi individuals suffered from cretinism, then how did they come to be together in the Dinaledi chamber of the Rising Star cave? One cannot help but feel a sense of the macabre about this death chamber. The Berger group prefer the explanation that the bodies of Homo naledi were deliberately put there by other Homo naledi, “over a long time, perhaps centuries”.133 Another explanation by evolutionists, from paleoanthropologist Curtis Marean, is that “if they date to the last 300,000 years, then it is plausible that early modern humans killed them and stashed them in the cave as part of a ritual”.62 Whilst aspects of their explanations are worth considering, their reasoning is based on a belief in evolution, and the notion that earlier humans were more ‘primitive’ in thought. From a biblical perspective humans never went through a primitive stage, having always been capable of intelligent thought and, after the Fall, of much evil also.
What if the Homo naledi individuals, being different because they were cretins, became vilified by, or ostracized from, their community because of their disease, perhaps blamed for some misfortune by a superstitious community? As a result, they could have been forced into the chamber, and left there to die. In a slight variant of the above, one wonders whether the presence of the specimens in the chamber under such unusual circumstances (i.e. no other large mammals, etc.) could not possibly point to some form of euthanasia (by abandoment in the cave—perhaps they were given poison to take whilst there) due to deformity/cretinism?
Another scenario could have involved them being sheltered together in the same area, away from the rest of the population, and the rest of the community migrating to a new area without them, or being wiped out in a conflict, and so the diseased individuals took refuge in the chamber, or were forced in there by the new arrivals, and somehow they ended up dying in there (whether by suicide or murder). Perhaps there was another way in, but that entry and exit point collapsed or was blocked, leaving them trapped inside the chamber. Looking at the photo of the fossil material in Figure 1 it seems as if the larger diameter long-bones (e.g., femurs) are fragmented, fairly systematically, whereas the smaller long-bones (e.g., phalanges) are less so. This is strongly indicative of cannibalism, where the larger bones of the limbs, such as the thigh bones, are favoured for breaking to suck out the bone marrow. This could indicate that the Dinaledi chamber was used as some sort of garbage disposal area, where human remains were disposed of after cannibalistic feasts or rituals elsewhere. Perhaps deformed humans were frequent victims of this ghoulish practice. Also, if the soil in the entire region were iodine-deficient, the entire community may have suffered from cretinism, and so the individuals in the chamber may have been representative of the population. The above scenarios are very speculative, and one could invent more scenarios, as one could with the Dmanisi Homo erectus specimens,85 but the truth is that no one really knows what happened.
References and notes
- Brown, P., Sutikna, T., Morwood, M.J., Soejono, R.P., Jatmiko, Saptomo E.W., and Due, R.A., A new small–bodied hominin from the late Pleistocene of Flores, Indonesia, Nature 431(7012):1055, 2004 | doi:10.1038/nature02999. Return to text
- Lordkipanidze, D., Ponce de Leόn, M.S., Margvelashvili, A., Rak, Y., Rightmire, G.P., Vekua, A. and Zollikofer, P.E., A complete skull from Dmanisi, Georgia, and the evolutionary biology of early Homo, Science, 342(6156):326–331, 2013 | doi: 10.1126/science.1238484. Return to text
- Berger, L.R. et al., Homo naledi, a new species of the genus Homo from the Dinaledi Chamber, South Africa, eLife 4:e09560, 2015 | doi:10.7554/eLife.09560. Return to text
- Berger, ref. 3, pp. 17–18. Return to text
- Dirks, P.H.G.M. et al., Geological and taphonomic context for the new hominin species Homo naledi from the Dinaledi Chamber, South Africa, eLife 4:e09561, 2015 | doi:10.7554/eLife.09561. Return to text
- Dirks, ref. 5, pp. 28–30. Return to text
- Dirks, ref. 5, pp. 1–2. Return to text
- Shreeve, J., Mystery man, National Geographic 228:39, October 2015. Return to text
- Homo naledi Fact Sheet: Questions and Answers, September 2015, wits.ac.za. Return to text
- Ambler, M., What to make of Homo naledi?, 22 September 2015, creation.com/homo-naledi. Return to text
- Berger, ref. 3, pp. 4–5. Return to text
- Callaway, E., Crowdsourcing digs up an early human species, Nature 525(7569):297–298, 2015 | doi:10.1038/nature.2015.18305. Return to text
- Schwartz, J.H., Tattersall, I. and Chi, Z., Comment on “A Complete Skull from Dmanisi, Georgia, and the Evolutionary Biology of Early Homo”, Science 344(6182):360, 2014 | doi: 10.1126/science.1250056. Return to text
- Ref. 9, p. 3. Return to text
- Stringer, C., The Many Mysteries of Homo naledi, eLife 4:e10627, 2015 | doi:10.7554/eLife.10627. Return to text
- Berger, ref. 3, p. 18. Return to text
- Berger, ref. 3, p. 22. Return to text
- Walker, A. and Shipman, P., The Wisdom of Bone, Phoenix, London, pp. 196–197, 1997. Return to text
- Tattersall, I., The Strange Case of The Rickety Cossack, Palgrave Macmillan, New York, NY, pp. 203–204, 2015. Return to text
- Tattersall, ref. 19, pp. 203, 2015. Return to text
- Prüfer, K., et al., The complete genome sequence of a Neanderthal from the Altai Mountains, Nature 505(7481):43–49, 2014 | doi: 10.1038/nature12886. Return to text
- Berger, ref. 3, p. 18. Return to text
- Berger, ref. 3, p. 22. Return to text
- Larson, S.G., Evolutionary Transformation of the hominin shoulder, Evol. Anthr. 16(5):172–187, 2007 | doi: 10.1002/evan.20149. Return to text
- Lordkipanidze, D. et al., Postcranial evidence from early Homo from Dmanisi, Georgia, Nature 449(7160): 305–310, 2007 | doi:10.1038/nature06134. Return to text
- Roach, N.T. and Richmond, B.G., Clavicle length, throwing performance and the reconstruction of the Homo erectus shoulder, J Hum Evol. 80:107–113, 2015 | doi: 10.1016/j.jhevol.2014.09.004. Return to text
- Aiello, L. and Dean, C., An Introduction To Human Evolutionary Anatomy, Academic Press, San Diego, pp. 353–355, 1990. Return to text
- Cartmill, M. and Smith, F.H., The Human Lineage, Wiley-Blackwell, New Jersey, p. 174, 2009. Return to text
- Larson, S.G., Evolution of the hominin shoulder: Early Homo, In: F.E. Grine, J.F. Fleagle and R.E. Leakey (Editors), The First Humans – Origin and Early Evolution of the Genus Homo, Springer, p. 68, 2009. Return to text
- Hawks, J., Is Homo naledijust a primitive version of Homo erectus?, September 2015, johnhawks.net. Return to text
- Berger, ref. 3, pp. 17–18. Return to text
- Berger, ref. 3, p. 22. Return to text
- Berger, ref. 3, p. 23. Return to text
- Ref. 8, p. 52. Return to text
- Richmond, B.G., Biomechanics of phalangeal curvature, J Hum Evol. 53(6):678–690, 2007 | PMID: 17761213. Return to text
- Kivell, T.L., Kibii, J.M., Churchill, S.E., Schmid, P. and Berger, L. R., Australopithecus sediba hand demonstrates mosaic evolution of locomotor and manipulative abilities, Science 333(6048):1411–1417, 2011 | doi: 10.1126/science.1202625. Supporting Online Material, pp. 16–17. Return to text
- Ref. 3, p. 17. Return to text
- Susman, R.L., New postcranial remains from Swartkrans and their bearing on the functional morphology and behavior of Paranthropus robustus, In: F.E. Grine (Editor), Evolutionary History Of The “Robust Australopithecines”, Aldine Transaction, New Brunswick, New Jersey, p. 157, 1988. Return to text
- Susman, R.L., de Ruiter, D. and Brain, C.K., Recently identified postcranial remains of Paranthropus and early Homo from Swartkrans Cave, South Africa, J Hum Evol. 41(6):607–629, 2001 | PMID: 11782111. Return to text
- Stern, Jr., J.T. and Susman, R.L., The locomotor anatomy of Australopithecus afarensis, Am J Phys Anthropol. 60(3):279–317 | PMID: 6405621. Return to text
- Stern, Jr., J.T., Jungers, W.L. and Susman, R.L., Quantifying phalangeal curvature: An empirical comparison of alternative methods, Am J Phys Anthropol. 97(1):1–10, 1995 | PMID: 7645670. Return to text
- Kivell T.L. et al., The hand of Homo naledi, Nat. Comm. 6:8431, 2015 | doi: 10.1038/ncomms9431. Return to text
- Karhausen, L., The Bleeding of Mozart, Xlibris, UK, p. 577, 2011. Return to text
- Holick, M.F., Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease, Am J Clin Nutr 80(suppl):1678S–1679S, 2004 | PMID: 15585788. Return to text
- Kivell, T.L., Evidence in hand: recent discoveries and the early evolution of human manual manipulation, Phil. Trans. R. Soc. B 370(1682):20150105, 2015 | doi: 10.1098/rstb.2015.0105. Return to text
- Larson, S.G., et al., Descriptions of the upper limb skeleton of Homo floresiensis, J Hum Evol. 57(5):555–570, 2009 | doi: 10.1016/j.jhevol.2008.06.007. Return to text
- Oxnard, C. Obendorf, P.J. and Kefford, B.J., Post-cranial skeletons of hypothyroid cretins show a similar anatomical mosaic as Homo floresiensis, PLoS ONE 5(9):e13018, 2010 | doi:10.1371/journal.pone.0013018. Return to text
- Shreeve, J., Mystery man, National Geographic 228:50, October 2015. Return to text
- Berger, ref. 3, p. 22. Return to text
- Wood, B. (Editor), Wiley-Blackwell Encyclopedia of Human Evolution, Volume I, John Wiley & Sons Ltd, West Sussex, UK, p. 403, 2011. Return to text
- Gruss, L.T., Scmitt, D., The evolution of the human pelvis: changing adaptations to bipedalism, obstetrics and thermoregulation, Phil. Trans. R. Soc. B 370(1663):20140063, 2015 | doi: 10.1098/rstb.2014.0063. Return to text
- Langdon, J.H., The Human Strategy, Oxford University Press, New York, pp. 117–118, 2005. Return to text
- Berger, ref. 3, pp. 17–18. Return to text
- Aiello, L. and Dean, C., An Introduction To Human Evolutionary Anatomy, Academic Press, San Diego, pp. 447, 1990. Return to text
- Jungers, W.L. et al., Descriptions of the lower limb skeleton of Homo floresiensis, J Hum Evol. 57(5):538–554, 2009 | doi: 10.1016/j.jhevol.2008.08.014. Return to text
- Oxnard, C. Obendorf, P.J. and Kefford, B.J., Post-cranial skeletons of hypothyroid cretins show a similar anatomical mosaic as Homo floresiensis, PLoS ONE 5(9):e13018, p. 1, 2010 | doi:10.1371/journal.pone.0013018. Return to text
- Oxnard, C., Ghostly Muscles, Wrinkled Brains, Heresies and Hobbits, World Scientific, Singapore, p. 319, 2008. Note that the word “lateral flair” was used, but it is presumed that “lateral flare” is what was meant. In another publication the same authors give the iliac blade orientation of cretins as lateral, same as Homo floresiensis and australopithecines. See Ref. 56, p. 3. Return to text
- Berger, ref. 3, p. 17. Return to text
- Berger, ref. 3, p. 22. Return to text
- Shreeve, J., Mystery man, National Geographic 228:57, October 2015. Return to text
- Harcourt-Smith, W.E.H. et al., The foot of Homo naledi, Nat. Comm. 6:8432, 2015 | doi: 10.1038/ncomms9432. Return to text
- Gibbons, A., New human species discovered, Science 349(6253):1149–1150, 2015 | doi: 10.1126/science.349.6253.1149. Return to text
- Berger, ref. 3, p. 17. Return to text
- DeSilva, J.M. and Throckmorton, Z.J., Lucy’s flat feet: The relationship between the ankle and rearfoot arching in early hominins, PLoS ONE, 5(12):e14432, 2010 | doi:10.1371/journal.pone.0014432. Return to text
- Ref. 9, p. 9. Return to text
- Harris, E.J. et al., Diagnosis and treatment of pediatric flatfoot, J Foot Ankle Surg. 43(6):341–373, 2004 | PMID: 15605048. Return to text
- Lee, M.S. et al., Diagnosis and treatment of adult flatfoot, J Foot Ankle Surg. 44(2):78–113, 2005 | PMID: 15768358. Return to text
- Lee, ref. 67, p. 78. Return to text
- Jungers, W.L., et al., Descriptions of the lower limb skeleton of Homo floresiensis, J Hum Evol. 57(5):538–554, 2009 | doi: 10.1016/j.jhevol.2008.08.014. Return to text
- Ref. 9, p. 9. Return to text
- Stern, Jr., J.T. and Susman, R.L., The locomotor anatomy of Australopithecus afarensis, Am J Phys Anthropol. 60(3):279–317, 1983 | PMID: 6405621. Return to text
- Lee, ref. 67, p. 6. Return to text
- Berger, ref. 3, p. 22. Return to text
- Berger, ref. 3, p. 18. Return to text
- Berger, ref. 3, p. 23. Return to text
- Berger, ref. 3, p. 21. Return to text
- Berger, ref. 3, p. 8. Return to text
- Wood, B. (Editor), Wiley-Blackwell Encyclopedia of Human Evolution, Volume I, John Wiley & Sons Ltd, West Sussex, UK, p. 398, 2011. Return to text
- Ruff, C. B. and Higgins, R., Femoral neck structure and function in early hominins. Am. J. Phys. Anthropol. 150(4):512–525, 2013 | doi: 10.1002/ajpa.22214. Return to text
- Ruff, ref. 79, See Figure 6a. Return to text
- Ruff, ref. 79, p. 516. Return to text
- Berger, ref. 3, pp. 5–7. Return to text
- Berger, ref. 3, p. 17. Return to text
- Randolph-Quinney, P.S., A new star rising: Biology and mortuary behaviour of Homo naledi. S Afr J Sci. 111(9/10):1–4, 2015 | http://dx.doi.org/10.17159/sajs.2015/a0122. Return to text
- Line, P., New Dmanisi skull threatens to bring the house down, 29 October 2013, creation.com/dmanisi. Return to text
- Hartley, R., Some bones to pick, timeslive.co.za, September 2015. Return to text
- Stringer, C., The Many Mysteries of Homo naledi, eLife 4:e10627, p. 2, 2015 | doi:10.7554/eLife.10627. Return to text
- Berger, ref. 3, p. 20. Return to text
- Berger, ref. 3, pp. 19–20. Return to text
- Shreeve, J., Mystery man, National Geographic 228:44, 49, October 2015. Return to text
- Schwartz, J.H., Why the Homo naledi discovery may not be quite what it seems, 10 September 2015, europe.newsweek.com. Return to text
- Schwartz, J.H., Tattersall, I. and Chi, Z., Comment on “A Complete Skull from Dmanisi, Georgia, and the Evolutionary Biology of Early Homo”, Science 344(6182):360, 2014 | doi: 10.1126/science.1250056. Return to text
- Berger, ref. 3, p. 2. Return to text
- Berger, ref. 3, p. 18. Return to text
- Berger, ref. 3, pp. 27–31. Return to text
- Bromage, T.G., McMahon, J.M., Thackeray, J.F., Kullmer, O., Hogg, R., Rosenberger, A.L., Schrenk, F. and Enlow, D.H., Craniofacial architectural constraints and their importance for reconstructing early Homo skull KNM-ER 1470, J Clin Pediatr Dent. 33(1):43–54, 2008 | PMID: 19093651. Return to text
- Leakey, R.E., Evidence for an advanced plio-pleistocene hominid from East Rudolf, Kenya, Nature 242(5398):447–450, 1973 | doi:10.1038/242447a0. Return to text
- Holloway, R.L., Human paleontological evidence relevant to language behavior, Hum Neurobiol. 2(3):105–114,1983 | PMID: 6421780. Return to text
- Bromage, ref. 96, p. 45. Return to text
- Bromage, ref . 96, p. 53. Return to text
- Line, P., Sorting ‘early’ Homo, J. Creation 27(1):14, 2013; creation.com/early-homo. Return to text
- Holden, C., Random Samples: New face for Kenya hominid, Science 316(5831):27, 2007. Return to text
- Spoor, F., Implications of new early Homo fossils from Ileret, east of Lake Turkana, Kenya, Nature 448(7154):688–691, 2007 | doi:10.1038/nature05986. Return to text
- Lordkipanidze, ref. 2, p. 327. Return to text
- Lordkipanidze, ref. 2, Supplementary Materials, Table S2A, p. 18. Return to text
- Gabunia, L. et al., Earliest Pleistocene hominid cranial remains from Dmanisi, Republic of Georgia: Taxonomy, geological setting and age, Science 288(5468):1019–1025, 2000 | doi: 10.1126/science.288.5468.1019. Return to text
- Lordkipanidze, ref. 2, p. 327. Return to text
- Holloway, R.L., Broadfield, D.C. and Yuan, M.S., The Human Fossil Record, Volume Three: Brain Endocasts—The Paleoneurological Evidence, John Wiley & Sons, Inc., Hoboken, New Jersey, p. 297, 2004. Return to text
- Wood, B. and Collard, M., The changing face of genus Homo, Evol. Anthropol. 8(6):195–207, 1999 | doi: 10.1002/(SICI)1520–6505(1999)8:6<195::AID-EVAN1>3.0.CO;2–2. Return to text
- Molnar, S., Races, Types, and Ethnic Groups, Prentice-Hall Inc., NJ, pp. 56–57, 1975. Return to text
- Schoenemann, P.T., Hominid brain evolution. In: D.R. Begun (Editor), A Companion to Paleoanthropology, Wiley-Blackwell, West Sussex, UK, pp. 144–145, 2013. Return to text
- Kubo, D., Kono, R.T. and Kaifu, Y., Brain size of Homo floresiensis and its evolutionary implications, Proc. R. Soc. B 280(1760):20130338, p. 1, 2013 | doi: 10.1098/rspb.2013.0338. Return to text
- Gauger, A., Science and Human Origins. In: Gauger, A., Axe, D. and Luskin, C., Science and Human Origins, Discovery Institute Press, Seattle, p. 26, 2012. Return to text
- Sanford, J.C., Genetic Entropy, Fourth Edition, FMS Publications, 2014. Return to text
- Sanford, ref. 114., p. 127. Return to text
- Berger, ref. 3, p. 17. Return to text
- Martin, G., Bones of contention: Why Cal paleo expert is so skeptical that Homo naledi is new species, 1 October 2015, alumni.berkeley.edu. Return to text
- Skoyles, J.R., Human evolution expended brains to increase expertise capacity, not IQ, Psycoloquy 10(002), 1999, cogsci.ecs.soton.ac.uk. Return to text
- Aiello, L. and Dunbar, R., Neocortex size, group size and the evolution of language, Current Anthropology 34(2):184–193, 1993 | http://www.jstor.org/stable/2743982. The formula derived by the authors is: Log10(B) = 3.015 + 0.986 Log10(C), where B is the total brain size (brain volume) in mm3 and C is the internal cranial capacity in cubic centimeters (cc). Return to text
- Line, P., Explaining robust humans, J. Creation 27(3):64–71, 2013; creation.com/explaining-robust-humans. Return to text
- Oxnard, C., Ghostly Muscles, Wrinkled Brains, Heresies and Hobbits, World Scientific, Singapore, pp. 303, 342, 2008. Return to text
- Dobson, J.E., The iodine factor in health and evolution, Geogr Rev 88(1):3–28, 1998 | doi: 10.1111/j.1931–0846.1998.tb00093.x. Return to text
- Obendorf, P.J., Oxnard, C.E. and Kefford, B.J., Are the small human-like fossils found on Flores human endemic cretins?, Proc. R. Soc. B 275(1640):1287–1296, 2008 | doi: 10.1098/rspb.2007.1488. Return to text
- Berger, L.R, Churchill, S.E., De Klerk, B. and Quinn, R.L., Small-bodied humans from Palau, Micronesia, PLoS ONE 3(3):e1780, 2008 | doi:10.1371/journal.pone.0001780. Return to text
- Brown, P., Sutikna, T., Morwood, M.J., Soejono, R.P., Jatmiko, Saptomo E.W. and Due, R.A., A new small-bodied hominin from the late Pleistocene of Flores, Indonesia, Nature 431(7012):1055–1061, 2004 | doi:10.1038/nature02999. Return to text
- Larson, S.G. et al., Homo floresiensis and the evolution of the hominin shoulder, J Hum Evol. 53(6):718–31, 2007 | PMID: 17692894. Return to text
- Larson, S.G., et al., Descriptions of the upper limb skeleton of Homo floresiensis, J Hum Evol. 57(5):555–570, 2009 | doi: 10.1016/j.jhevol.2008.06.007. Return to text
- Jungers, ref. 69, p. 538. Return to text
- Oxnard, ref. 121, p. 319. Return to text
- Oxnard, C., Obendorf, P.J., Kefford, B.J., Dennison, J., More on the Liang Bua finds and modern human cretins, Homo. 63(6):407–12, 2012 | doi: 10.1016/j.jchb.2012.09.005. Return to text
- Oxnard, ref. 121, p. 320. Return to text
- Oxnard, ref. 121, p. 342. Return to text
- Shreeve, J., Mystery man, National Geographic 228:53, October 2015. Return to text
















Readers’ comments
Comments are automatically closed 14 days after publication.